Table 3. Optimization of Pd–NiXantphos Catalyzed DCCP of 1a with 2aa.
| entry | 1a:2a | Pd (mol %) | solvent | concentration (M) | yield (%)b |
|---|---|---|---|---|---|
| 1 | 1.2:1.0 | 10 | CPME | 0.1 | 87 |
| 2 | 1.2:1.0 | 10 | DME | 0.1 | 61 |
| 3 | 1.2:1.0 | 10 | 2-MeTHF | 0.1 | 78 |
| 4 | 1.2:1.0 | 10 | THF | 0.1 | >95 |
| S | 1.2:1.0 | 10 | dioxane | 0.1 | 25 |
| 6 | 1.2:1.0 | 10 | MTBE | 0.1 | 10 |
| 7 | 1.2:1.0 | 5 | THF | 0.1 | 79 |
| 8 | 1.2:1.0 | 5 | THF | 0.2 | 58 |
| 9c | 1.2:1.0 | 5 | THF | 0.2 | 76 |
| 10 | 1.0:2.0 | 5 | THF | 0.2 | >95 (99)d |
| 11 | 1.0:2.0 | 2.5 | THF | 0.2 | (81)d |
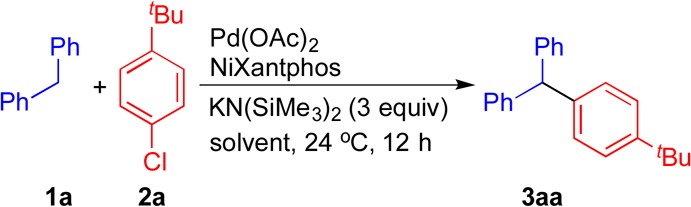
Reactions conducted on a 0.1 mmol scale using 1a, 2a, and 3 equiv of KN(SiMe3)2 at 24 °C.
Yield determined by 1H NMR spectroscopy of the crude reaction mixture.
5 mol % the methanesulfonate precatalyst 4 was used in place of Pd(OAc)2/NiXantphos.
Isolated yield after chromatographic purification.
