Table 4. Scope of Aryl Chlorides in Pd–NiXantphos-Catalyzed DCCP with 1aa.
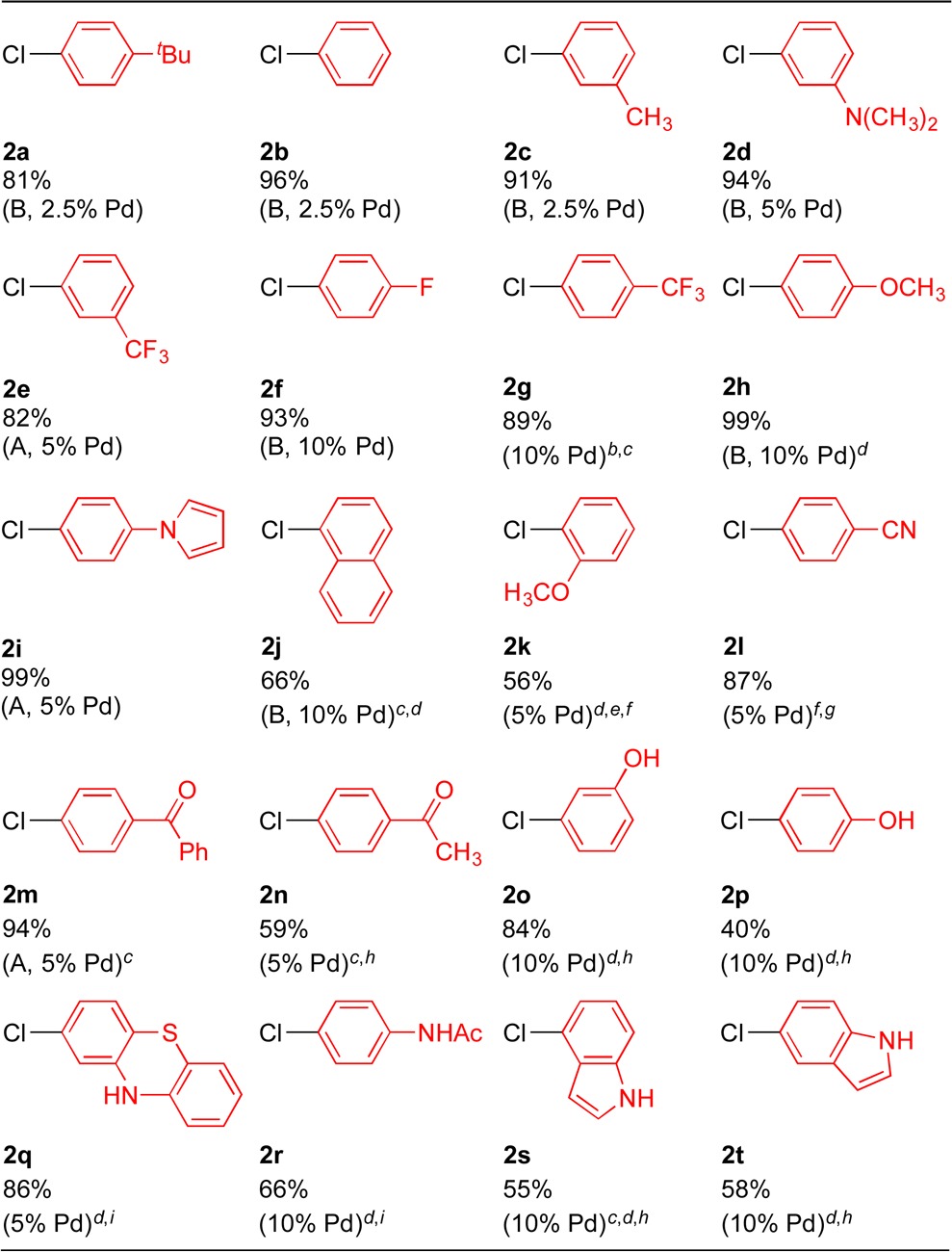
Reactions conducted on a 0.1 mmol scale using 1.2 equiv of 1a, 3 equiv of KN(SiMe3)2, and 1 equiv of 2 (conditions A) or 1 equiv of 1a, 3 equiv of KN(SiMe3)2, and 2 equiv of 2 (conditions B) using 2.5–10 mol % Pd(OAc)2 and NiXantphos (Pd:L = 1:2) in THF at 0.2 M at 24 °C. Isolated yield after chromatographic purification.
1a:KN(SiMe3)2:2 = 4:2:1.
CPME was used as solvent.
80 °C.
1a:KN(SiMe3)2:2 = 1:3:3.
2-MeTHF was used as solvent.
1a:KN(SiMe3)2:2 = 1.2:4:1.
1a:KN(SiMe3)2:2 = 3:4:1.
1a:KN(SiMe3)2:2 = 1:5:2.
