Fecal microbiota transplant is increasingly used to treat recurrent or relapsing Clostridium difficile infection. In this randomized controlled study, using a frozen inoculum from unrelated donors was safe and effective, whether administered by nasogastric tube or by colonoscopy.
Keywords: fecal microbiota transplant, Clostridium difficile, microbiome, frozen inoculum
Abstract
Background. Recurrent Clostridium difficile infection (CDI) with poor response to standard antimicrobial therapy is a growing medical concern. We aimed to investigate the outcomes of fecal microbiota transplant (FMT) for relapsing CDI using a frozen suspension from unrelated donors, comparing colonoscopic and nasogastric tube (NGT) administration.
Methods. Healthy volunteer donors were screened and a frozen fecal suspension was generated. Patients with relapsing/refractory CDI were randomized to receive an infusion of donor stools by colonoscopy or NGT. The primary endpoint was clinical resolution of diarrhea without relapse after 8 weeks. The secondary endpoint was self-reported health score using standardized questionnaires.
Results. A total of 20 patients were enrolled, 10 in each treatment arm. Patients had a median of 4 (range, 2–16) relapses prior to study enrollment, with 5 (range, 3–15) antibiotic treatment failures. Resolution of diarrhea was achieved in 14 patients (70%) after a single FMT (8 of 10 in the colonoscopy group and 6 of 10 in the NGT group). Five patients were retreated, with 4 obtaining cure, resulting in an overall cure rate of 90%. Daily number of bowel movements changed from a median of 7 (interquartile range [IQR], 5–10) the day prior to FMT to 2 (IQR, 1–2) after the infusion. Self-ranked health score improved significantly, from a median of 4 (IQR, 2–6) before transplant to 8 (IQR, 5–9) after transplant. No serious or unexpected adverse events occurred.
Conclusions. In our initial feasibility study, FMT using a frozen inoculum from unrelated donors is effective in treating relapsing CDI. NGT administration appears to be as effective as colonoscopic administration.
Clinical Trials Registration. NCT01704937.
Recurrent and refractory Clostridium difficile infection (CDI) is a growing medical concern, with a recent dramatic increase in the number of patients globally [1–4]. In the United States, the incidence of CDI has tripled over the last 15 years [3]. Response to standard antimicrobial therapy with oral vancomycin or metronidazole is suboptimal, with CDI recurring in up to 30% of individuals treated for a first episode. After 2 or more episodes of CDI, the estimated risk for subsequent recurrence exceeds 60% with antimicrobial therapy [3, 5–8]. Often, patients with recurrent CDI are treated with prolonged administration of oral vancomycin with tapering of the medication over many months, but this approach is poorly studied. The emergence of a virulent strain of the organism (NAP1/BI/027) has been associated with even higher rates of treatment failure [9, 10]. The consequences of recurrence can be devastating, resulting in life-threatening illness, frequent hospitalizations, and possible surgical interventions. In addition to individual morbidity and mortality, CDI taxes the medical system by requiring patient cohorting, leading to bed closures, delay of discharge, and additional contact precautions.
Although the illness is toxin-mediated, overgrowth of the organism in the setting of dysbiosis is thought to be a key inciting event. Failure to reconstitute normal flora was shown to be a factor in severe, recurrent, and prolonged illness [11]. Fecal microbiota transplant (FMT)—reconstitution of normal flora by a stool transplant from a healthy individual—has been a successful therapeutic approach to recurrent/refractory CDI in animal studies [12], numerous case series [13–18], and, more recently, a single randomized clinical trial [19]. Even though an overall CDI resolution rate of about 90% has repeatedly been reported in published reviews and meta-analyses [20–23], practical and aesthetic barriers have hindered the widespread use of FMT to date. Recruitment and screening of donors is a lengthy process associated with significant costs, thus preventing the use of FMT in acute situations. Establishing a repository of prescreened frozen donor stools could make this treatment available for a wider population. Furthermore, many questions remain regarding the optimal protocol donor screening, sample processing, route of administration, and amount of fecal material instilled.
In the current study, we aimed to investigate the clinical outcomes of FMT for refractory or relapsing CDI using a frozen suspension from unrelated donors by both upper and lower gastrointestinal routes.
METHODS
This was an open-label randomized controlled trial evaluating the efficacy of FMT in treating relapsing or recurrent CDI in a pilot cohort of 20 patients, comparing colonoscopic and nasogastric tube (NGT) administration. The study was approved by the Partners Human Research Committee as well as by the US Food and Drug Administration (FDA) (Investigational New Drug application number 15199) and registered at Clinical Trials.gov (NCT01704937). Candidates were recruited by referrals from colleagues at Partners HealthCare (of which Massachusetts General Hospital is a founding member). All adult participants provided written informed consent after a clinical meeting with a physician investigator providing information about potential risks and benefits of the procedure. Children aged 7 years or older provided assent, in addition to parental informed consent. Participants were allocated to treatment arms by computer-generated randomization in blocks of 4.
Study Population and Settings
The study was conducted at Massachusetts General Hospital. Included were subjects aged 7–90 with refractory or recurrent CDI, as defined in consensus guidelines [14] by a relapse of CDI after having at least 3 episodes of mild-to-moderate CDI and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic, OR at least 2 episodes of severe CDI resulting in hospitalization and associated with significant morbidity. Active CDI was defined as diarrhea (>3 loose stools per day) with a positive stool test for C. difficile toxin. Our hospital laboratory performs an initial toxin/glutamate dehydrogenase (GDH) enzyme-linked immunosorbent assay, followed by polymerase chain reaction only if the GDH test is positive or indeterminate, and does not routinely test for the NAP-1/B1/207 strain. Exclusion criteria included presence of anatomic contraindication to NGT or colonoscopy, delayed gastric emptying syndrome, recurrent aspirations, pregnancy, significantly compromised immunity (immunosuppressive medications, recent chemotherapy, decompensated liver cirrhosis, advanced human immunodeficiency virus [HIV]/AIDS [CD4 count <250 cells/µL], neutropenia with absolute neutrophil count <1000/µL, recent bone marrow transplant, or other cause of severe immunodeficiency), and having a history of significant allergy to foods not excluded from the donor diet. Stable oral prednisone treatment up to 40 mg daily was allowed.
Donor Screening
Donors were healthy, nonpregnant adults 18–50 years of age, on no medications, with a normal body mass index (18.5–25 kg/m2). Volunteers were excluded for any significant past medical history (with the exception of resolved traumatic injury) or any use of antibiotics in the preceding 6 months. Candidates were initially screened using the American Association of Blood Banks donor questionnaire for exposure to infectious agents [24], then underwent physical examination and general laboratory screening tests (within 30 days of donations), including complete blood count with differential, renal function and electrolytes, complete liver function tests, albumin and total protein, lipid profile, high-sensitivity C-reactive protein, fluorescent antinuclear antigen, and fecal occult blood testing. All results had to be within normal range for age and sex. Donor feces were screened for enteric bacterial pathogens including rotavirus, Listeria monocytogenes, Vibrio cholerae, Escherichia coli O157, ova and parasites (including general microscopy, acid-fast staining, and/or antigen testing for Giardia, Cryptosporidium, Isospora, and Microsporidia), C. difficile, and Helicobacter pylori antigen. Blood was screened for antibodies to hepatitis A, B, and C; HIV; and Treponema pallidum within 2 weeks of donations. The volunteers were asked to refrain from eating common allergens within 5 days of stool donation (tree nuts, eggs, peanuts, shellfish) but otherwise not to alter their diets. At the time of donation they had an interim health query for febrile, systemic, and gastrointestinal symptoms and were deferred for any change in health status. Finally, all donations were escrowed for an additional 4 weeks, to allow retesting of donors for HIV and hepatitis B and C prior to clinical use of the inoculum.
Preparation of Frozen Inocula
Donors were asked to take a dose of milk of magnesia the day before fecal collections to facilitate manipulation of the sample. A fecal suspension was generated in normal saline without preservatives, using a commercial blender. Materials were sequentially passed through 4 sieves to remove particulate material. The final slurry was concentrated 3-fold by centrifugation and then resuspended in sterile saline with 10% glycerol added as a bacterial cryoprotectant. Inocula were then frozen at −80°C pending use. The work of Hamilton et al [25] was used as a guide for fecal manipulation, with the exception that all processes were carried out under ambient air, not nitrogen. Each sieved inoculum was calculated at the conclusion of the project to have been derived from approximately 41 g of fecal material. Inocula used in this study were stored frozen for up to 156 days (range, 29–156 days). Frozen material was thawed in a 37°C water bath, and then kept on ice until delivery.
Study Procedures
Patients were required to discontinue all antibiotics at least 48 hours prior to the procedure (Supplementary Figure 1). Subjects assigned to colonoscopic administration underwent a standard bowel preparation with 4 liters of polyethylene glycol electrolyte solution, followed by endoscopic administration to the right colon of 90 cc thawed inoculum. This amount of fecal material was further diluted to 250 cc for adults and 160 cc for pediatric patients. Patients were asked to retain the material as long as possible after the procedure and were given a single oral dose of loperamide at the time of the procedure. Subjects assigned to NGT delivery of FMT were prescribed 2 mg/kg/day, up to 20 mg, of omeprazole orally for 48 hours prior to the procedure. An age- and size-appropriate NGT was inserted, proper positioning in the stomach was documented by radiography, and 90 cc of inoculum was administered. In these patients the inoculum was not further diluted, to minimize risk of vomiting and aspiration. The NGT was removed promptly after administration and subjects were asked to drink a glass of water to facilitate dilution of stomach contents and transit into the small intestine.
Patients in both study arms who showed no improvement in diarrheal symptoms were offered a second FMT by their preferred route of administration. To minimize potential infectious exposures, inoculum from the same donor was used for the repeat administration.
Patients in both groups were followed with structured questionnaires administered on days 1, 2, 3, 7, 14, and 21, and at 2 and 6 months after the procedure (primarily by phone). Questionnaires recorded stool frequency and consistency, general well-being on a standardized health score, rating of gastrointestinal symptoms, medication use, and weight changes, and elicited possible adverse events by use of a modification of the Common Terminology Criteria for Adverse Events version 3.0 [26] approved by the FDA and institutional review board (IRB).
Outcomes
The primary endpoint was clinical resolution of diarrhea off antibiotics for C. difficile, without relapse within 8 weeks. For patients who required a second treatment dose, follow-up was calculated starting at the time of the second administration. Resolution of diarrhea was defined as <3 bowel movements per 24 hours. Secondary endpoints included improvement in subjective well-being per standardized questionnaire and presence of adverse events.
Data Analysis
Continuous variables are presented as mean and standard deviation (SD) or median and interquartile range (IQR). Categorical variables are presented as number and percentage of patients within each treatment group. Patient characteristics at baseline were compared between the 2 treatment groups to estimate the efficacy of randomization. The Mann-Whitney test was used for comparisons of continuous variables (patient characteristics and outcomes) between the 2 treatment groups and Fisher exact test for comparisons of categorical variables. Outcomes were analyzed according to the intention-to-treat principle, with imputation of data by the last outcome carried forward. Mixed-model analysis of variance was used to estimate difference in outcomes between the 2 treatment groups over the study period.
All statistical tests were 2-sided; a P value < .05 was considered statistically significant. Data were analyzed using SPSS statistical software version 21 (SPSS Inc, Chicago, Illinois).
Analysis of Fecal Microbiota
A donor sample was collected at time of donation. Recipients provided stool samples before FMT, weekly for 3 weeks and then at 2 and 6 months. All fecal samples were stored at −80°C. DNA was extracted, and the V4 region of the 16S gene was sequenced using an Illumina MiSeq (Illumina, San Diego, California) as described previously [27]. The Shannon Diversity Index was computed for each sample and a custom python script was used to create summary plots illustrating the relationship between clinically relevant groupings and the diversity observed in the microbiome. We used the Shannon diversity index as our primary measure of diversity because it takes into account both abundance and evenness of species present in the community and has been shown to most robustly accommodate the variation in sampling depth [28]. See Supplementary Appendix 1 for detailed methods.
RESULTS
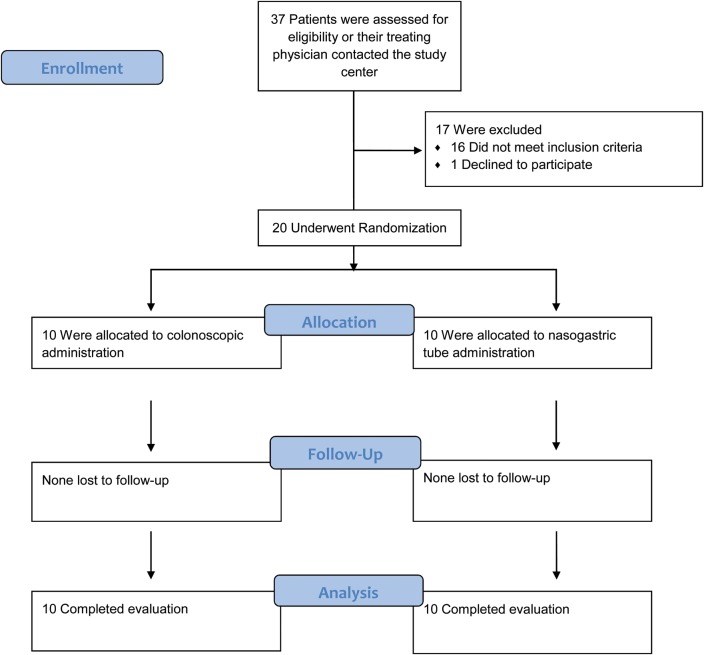
From December 2012 through May 2013, a total of 20 patients were randomly assigned to receive FMT via colonoscopy or NGT (Figure 1). Baseline characteristics were comparable between groups (Table 1).
Figure 1.
Enrollment and follow-up.
Table 1.
Select Baseline Characteristics of Study Population Stratified by Treatment Group
| Characteristic | Initial Colonoscopy | Nasogastric Tube | P Value |
|---|---|---|---|
| Age, ya | 50.4 ± 28.8 | 58.6 ± 19.6 | .739 |
| Female sexb | 6 (60) | 5 (50) | 1.00 |
| Time since initial CDI, mob | 7 (3–34) | 12 (3–66) | 1.00 |
| Hospital-acquired CDIb | 2 (20) | 3 (30) | 1.00 |
| Number of CDI recurrences prior to FMTc | 4 (2–7) | 5 (3–16) | .42 |
| Previous vancomycin taperb | 9 (90) | 10 (100) | 1.00 |
| Previous use of fidaxomicinb | 5 (50) | 7 (70) | .64 |
| Hospital admissions in the past due to CDIb | 6 (60) | 7 (70) | 1.00 |
| Inpatient at time of FMTb | 2 (20) | 3 (30) | 1.00 |
| No. of bowel movements 1 d prior to FMTc | 6 (4–13) | 7 (5–13) | .43 |
| Health status 1 d prior to FMTc | 5 (2–7) | 4 (1–10) | .21 |
Abbreviations: CDI, Clostridium difficile infection; FMT, fecal microbiota transplant.
a Mean ± standard deviation.
b No. (%).
c Median (range).
Donors
Of 37 candidates who responded to our call for volunteers, 12 passed the initial screening and underwent a full donor workup. Seven were excluded from donating based on abnormal screening laboratory results: 4 with positive antinuclear antibodies, 1 with elevated bilirubin, 1 with mild neutropenia, and 1 with eosinophilia. The remaining 5 donors provided 3 stool samples each, which were used to generate 25 infusions used in 20 study patients.
Primary Outcome
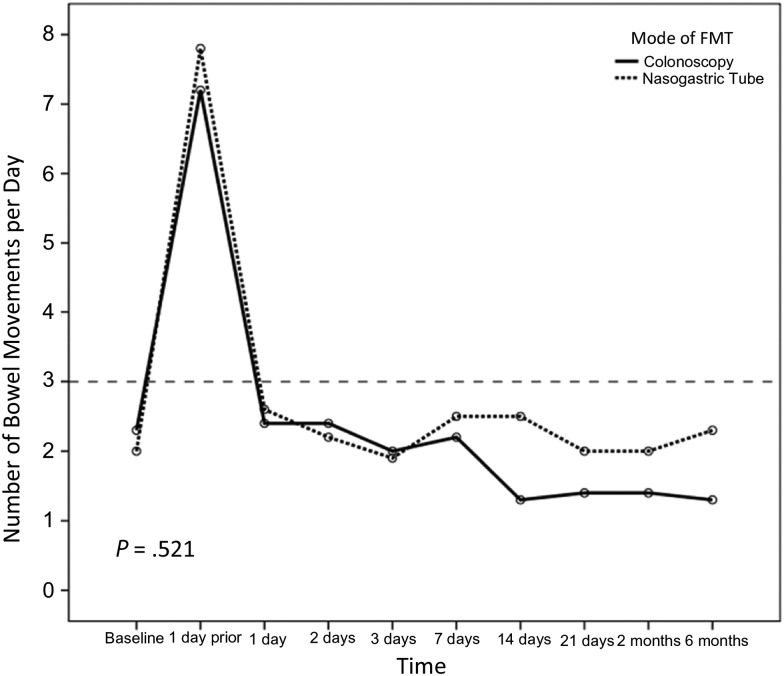
Of 20 patients in both study arms, 14 were cured after the first infusion of donor feces (70%): 8 in the colonoscopy group (80%) and 6 in the NGT group (60%; P = .628). One patient in the NGT arm refused subsequent retreatment. The remaining 5 patients were given a second infusion at a mean of 4.9 days (SD, 2.1 days) after the first procedure, using feces from the same donor who provided the initial inoculum. Per protocol, patients could choose the route of delivery for retreatment; and all 5 requested NGT administration. Four patients obtained cure after the second inoculation, resulting in an overall cure rate of 90% (80% in the NGT group and 100% in the initial colonoscopy group; P = .53). No patient relapsed within the predetermined 8-week follow up period after initial cure. Daily number of bowel movements changed from a median of 6 (IQR, 5–10) and 7 (IQR, 6–10) in the colonoscopy and NGT groups, respectively, the day prior to FMT (P = .436) to 1 (IQR, 1–1) and 2 (IQR, 1–2), respectively, 8 weeks after the infusion (P = .165; Figure 2).
Figure 2.
Mean number of daily bowel movements (BMs) in both study arms. Baseline represents reported BMs prior to contracting Clostridium difficile as reported by the patients. Six-month follow-up data are missing from 3 patients in the nasogastric tube group and 2 patients in the colonoscopy group. Abbreviation: FMT, fecal microbiota transplant.
Secondary Outcomes
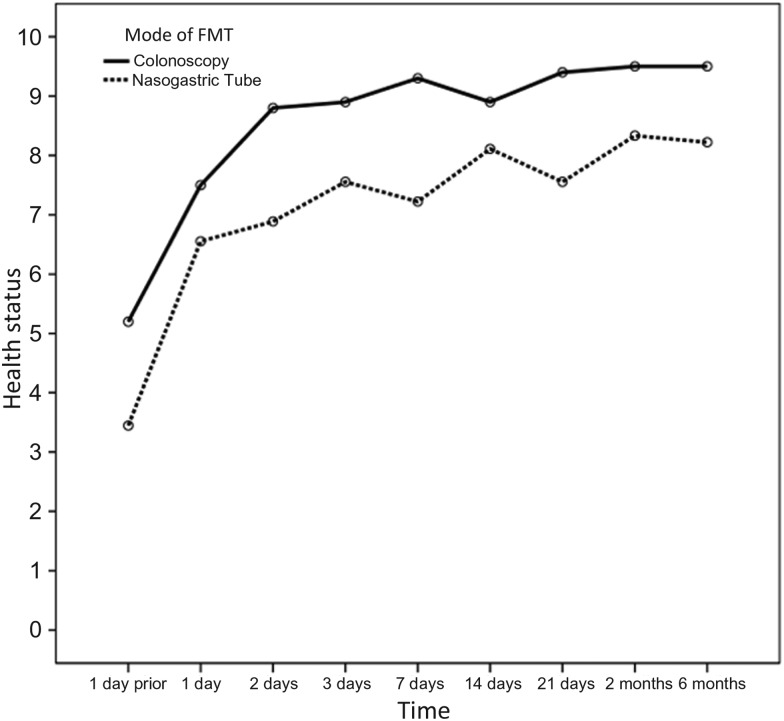
Self-reported health rating using a standardized questionnaire scale of 1–10, with 1 being the lowest and 10 being “your best recent health baseline,” increased over the study period from a median of 5 (IQR, 3–6) and 4 (IQR, 2–5) in the colonoscopy and NGT groups, respectively, the day prior to FMT (P = .436) to 8 (IQR, 7–10) and 7 (IQR, 5–8), respectively, 8 weeks after the infusion (Figure 3). The colonoscopy group had consistently higher health scores, accounted for by a higher reported score at day −1. When analyzing the absolute increment in scores, the groups did not differ (P = .51).
Figure 3.
Mean scores of subjective well-being over time as reported using a standardized questionnaire with a scale of 1–10, 1 being the lowest. The colonoscopy group had consistently higher scores, accounted for by a mean higher reported score at day –1. When analyzing the absolute increment in health scores, the groups did not differ (P = .51). Abbreviation: FMT, fecal microbiota transplant.
Adverse events deemed likely to be related included mild abdominal discomfort and bloating in 4 patients (20%). One child treated colonoscopically had a transient fever of 38.8°C on day 2 that resolved spontaneously. There were several serious adverse events that were assessed as unrelated by the investigators and IRB, and reflect the relatively poor health of many individuals with recurrent CDI. One patient died 12 weeks after the procedure while hospitalized secondary to an acute exacerbation of chronic obstructive pulmonary disease, including bleb rupture requiring intubation and chest tube. Although she was treated for several weeks with parenteral broad-spectrum antimicrobials, her CDI did not recur. Another patient died of metastatic laryngeal cancer 21 weeks after the procedure. A third patient was diagnosed with adenocarcinoma of the esophagus. A fourth patient, treated by the upper gastrointestinal route, was hospitalized for Fournier gangrene.
Fecal Microbiota
Fourteen stool samples from 4 donors and 65 samples from 19 recipients (21 pre-FMT and 44 at different time points after FMT) were analyzed. The Shannon diversity index of fecal microbiota obtained from recipients evaluated prior to FMT was consistently low (mean, 2.52 [SD, 0.77]) and increased after FMT (mean, 3.82 [SD, 0.74]) to a diversity level comparable to that of the donors (mean, 4.20 [SD, 0.51], P < .001 for the difference between pre- and post-FMT; P = .53 for the difference between post-FMT and donor stool) as shown in Figure 4. This level persisted over time, and there was no significant difference between the diversity index in stool samples obtained in the first week after the procedure and those obtained up to 6 months later (P = .11; Supplementary Appendix). The route of administration made no difference in the mean Shannon diversity index obtained after FMT (mean, 3.79 [SD, 0.64] in the colonoscopy group vs 3.84 [SD, 0.84] in the NGT group, P = .245; Figure 5). The microbiota composition and trajectories after FMT can be viewed in Supplementary Figures 3 and 4 in the Supplementary Appendix.
Figure 4.
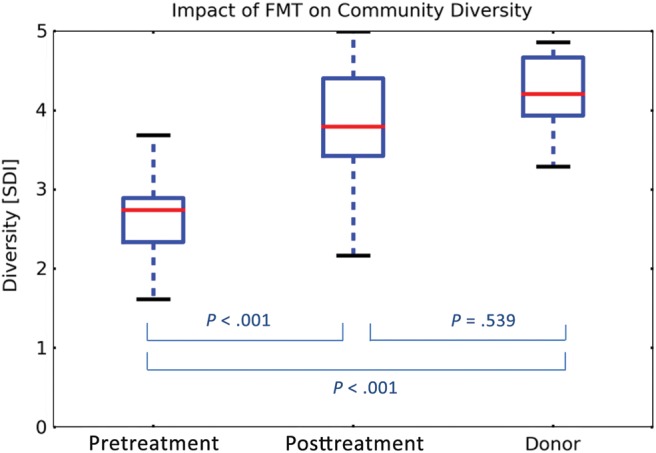
Microbiota diversity in fecal samples obtained from fecal microbiota transplant recipients before and after the procedure, as compared with the donors, expressed by the Shannon diversity index. The box-and-whisker plots indicate interquartile ranges (boxes), medians (red horizontal lines), and range (whiskers). Abbreviations: FMT, fecal microbiota transplant; SDI, Shannon diversity index.
Figure 5.
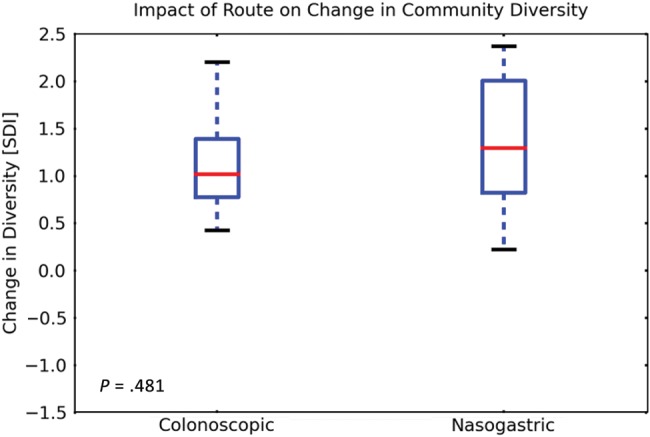
Microbiota diversity in fecal samples obtained from fecal microbiota transplant recipients before and after the procedure, stratified by treatment route and expressed by the Shannon diversity index. The box-and-whisker plots indicate interquartile ranges (boxes), medians (red horizontal lines), and range (whiskers). Abbreviation: SDI, Shannon diversity index.
DISCUSSION
In this small randomized controlled feasibility study, we demonstrated that infusion of unrelated frozen donor stools is efficacious in treating patients with relapsing/recurring CDI with an overall cure rate of 90% at 8 weeks. Furthermore, NGT seems to be a viable route of administration for the inoculum, a distinct advantage in elderly and/or debilitated patients who are prone to this condition who may not tolerate a colonoscopy or the sedation associated with the procedure. These data are especially encouraging in view of our study population, consisting of patients with at least 3 recurrences of CDI or 2 episodes of CDI resulting in hospitalization, in which the reported cure rate with standard antimicrobial treatment falls to <30% [7]. Moreover, 95% of patients had been treated with previous prolonged vancomycin tapers, and 70% of participants had been treated with fidaxomicin in the past, even further lowering the likelihood of obtaining cure with standard antimicrobial treatment. One 89-year-old patient with refractory disease had 16 documented episodes of CDI in the preceding 15 months, including 4 regular admissions and 2 admissions to the intensive care unit. She was cured with 2 inocula and has been asymptomatic off treatment for 12 months.
Interestingly, of the 2 patients in our study for whom treatment failed, one refused a second treatment dose after the initial inoculum had no curative effect. Unbeknown to us, we later learned that this patient self-administered homemade fecal enemas daily for a week, using unprocessed stool from his roommate. He subsequently reported feeling well and being completely asymptomatic, but as per our study definitions he was considered to have failed treatment. This example also brings to light the potential hurdles associated with regulating a readily available “biologic therapeutic,” as can also be evidenced by numerous “how to” manuals published on the Internet.
Although most of our patients were elderly, reflecting the main population in whom CDI develops, the mean age of our participants was only 54, influenced by the fact that we included 3 children in our study. This inclusion is important in view of the recent increase in the number of pediatric cases of CDI, including a growing population of children with recurrent/refractory disease [1, 29, 30]. All 3 pediatric patients were cured after administration of a single inoculum.
Since completing administration of FMT to the 20 study subjects, we have performed an additional 11 “expanded access” clinical administrations of FMT using frozen inocula from unrelated donors with a success rate of 90.9%. All were delivered via NGT.
Whereas a previous study demonstrated the superiority of FMT over standard antimicrobial treatment [19], the authors examined instillation of fresh donor stools. This strategy has several potential disadvantages, including the need for maintaining a readily available pool of donors, maintaining updated medical screening of donors, and, finally, the challenging logistics of obtaining the stool sample, processing the inoculum, and delivering the FMT within a limited time frame. The use of frozen inocula addresses many of these obstacles by allowing identification and screening of donors ahead of time and establishment of a bank of preprocessed and vetted material that is readily available on short notice. The banking of donor stools also allows the added safety of following donors for a period of time and retesting for infectious diseases that could potentially have been latent at the time of donation, prior to administration of the inoculum. The optimal “shelf life” of the inoculate is still unclear, but in our study the longest an inoculum was stored prior to clinical use was 156 days (mean, 79.3 days).
Despite numerous reports of successful resolution of CDI by FMT, the treatment has yet to become an available therapeutic option for many patients. This lack of availability not only deprives patients of the potential benefits of the procedure, but encourages patients to seek unregulated sources of information and alternative FMT providers, leading to treatment with unscreened fecal materials. As mentioned, this limitation can be partly explained by the logistical hurdles associated with the procedure [31]. Another inhibiting factor is the fact that the available data are mostly based on retrospective case series and include only a single randomized trial [19, 22, 23] to date, making practitioners cautious about adopting FMT as a viable treatment alternative. The variability in patient population, donor selection, inoculum preparation, and route and volume of administration all make pooling of published results challenging. Our study protocol (Supplementary Appendix) may be of value in standardizing FMT, and we hope, if adopted by others, will make future outcome data comparable between institutions.
A major limitation of our study is the small sample size. Nevertheless, our results were comparable to those in the literature reporting the use of fresh donor stools. Of particular importance is the fact that delivery of the inoculum through the upper gastrointestinal tract seems to be comparable to that of colonoscopic delivery, thus eliminating the need for sedation, anesthetic risks, and colonic “cleanout.” Possible vomiting and aspiration is a concern with upper gastrointestinal delivery, although we did not observe this complication in our study subjects or in 11 subsequent patients for care. We have now addressed this concern in part by further concentrating and encapsulating this inoculum in Capsugel DR hypromellose capsules, which resist dissolution in acidic environments. We are now studying oral delivery of frozen encapsulated material as the next logical step in making FMT more accessible to patients.
In conclusion, in our initial feasibility study, FMT using a frozen inoculum from unrelated donors was effective in treating relapsing CDI, even in patients with multiple recurrences. NGT administration appeared to be as effective as colonoscopic administration.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank Dr Tomer Ziv-Baran for his help with statistical analysis and Dr Ramnik Xavier and the Center for the Study of Inflammatory Bowel Disease (P30DK043351) for support of microbiome analysis.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, the National Institutes of Health (NIH), Harvard University, its affiliated academic healthcare centers, or its corporate contributors.
Financial support. The microbiome analysis was funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, NIH, Department of Health and Human Services (contract number HHSN272200900018C). I. Y. has received career support from Harvard Catalyst, The Harvard Clinical and Translational Science Center, funded by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH (award 8UL1TR000170-05), and financial contributions from Harvard University and its affiliated academic healthcare centers.
Potential conflicts of interest. M. B. S. is on the board of directors of OpenBiome, a 501(c)3 nonprofit aimed at expanding access to fecal microbiota preparations by providing screened, ready-to-use fecal material for clinical use. E. L. H. is the recipient of a sponsored research award from Seres Health, Cambridge, Massachusetts, to Massachusetts General Hospital for a clinical trial related to treatment of C. difficile colitis. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Khanna S, Baddour LM, Huskins WC, et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis. 2013;56:1401–6. doi: 10.1093/cid/cit075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarvis WR, Schlosser J, Jarvis AA, Chinn RY. National point prevalence of Clostridium difficile in US health care facility inpatients, 2008. Am J Infect Control. 2009;37:263–70. doi: 10.1016/j.ajic.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932–40. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 4.Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile infection in children. JAMA Pediatr. 2013;167:567–73. doi: 10.1001/jamapediatrics.2013.441. [DOI] [PubMed] [Google Scholar]
- 5.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 6.Pépin J, Routhier S, Gagnon S, Brazeau I. Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis. 2006;42:758–64. doi: 10.1086/501126. [DOI] [PubMed] [Google Scholar]
- 7.Pépin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am J Gastroenterol. 2007;102:2781–8. doi: 10.1111/j.1572-0241.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 8.Vardakas KZ, Polyzos KA, Patouni K, Rafailidis PI, Samonis G, Falagas ME. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidence. Int J Antimicrob Agents. 2012;40:1–8. doi: 10.1016/j.ijantimicag.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–36. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 10.Petrella LA, Sambol SP, Cheknis A, et al. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin Infect Dis. 2012;55:351–7. doi: 10.1093/cid/cis430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–8. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 12.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8:e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:580–5. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 14.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–9. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–87. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 16.Brandt LJ, Reddy SS. Fecal microbiota transplantation for recurrent clostridium difficile infection. J Clin Gastroenterol. 2011;45(suppl):S159–67. doi: 10.1097/MCG.0b013e318222e603. [DOI] [PubMed] [Google Scholar]
- 17.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46:145–9. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- 18.Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–6. doi: 10.1053/j.gastro.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 19.Van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 20.Glauser W. Risk and rewards of fecal transplants. CMAJ. 2011;183:541–2. doi: 10.1503/cmaj.109-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo B, Harstall C, Louie T, Veldhuyzen van Zanten S, Dieleman LA. Systematic review: faecal transplantation for the treatment of Clostridium difficile-associated disease. Aliment Pharmacol Ther. 2012;35:865–75. doi: 10.1111/j.1365-2036.2012.05033.x. [DOI] [PubMed] [Google Scholar]
- 22.Karadsheh Z, Sule S. Fecal transplantation for the treatment of recurrent Clostridium difficile infection. N Am J Med Sci. 2013;5:339–43. doi: 10.4103/1947-2714.114163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–8. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 24.Fridey JL, Townsend MJ, Kessler DA, Gregory KR. A question of clarity: redesigning the American Association Of Blood Banks blood donor history questionnaire—a chronology and model for donor screening. Transfus Med Rev. 2007;21:181–204. doi: 10.1016/j.tmrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–7. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 26.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 27.Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preheim SP, Perrotta AR, Friedman J, et al. Computational methods for high-throughput comparative analyses of natural microbial communities. Methods Enzymol. 2013;531:353–70. doi: 10.1016/B978-0-12-407863-5.00018-6. [DOI] [PubMed] [Google Scholar]
- 29.Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile infection in children. JAMA Pediatr. 2013;167:567–73. doi: 10.1001/jamapediatrics.2013.441. [DOI] [PubMed] [Google Scholar]
- 30.Village EG. Clostridium difficile infection in infants and children. Pediatrics. 2013;131:196–200. doi: 10.1542/peds.2012-2992. [DOI] [PubMed] [Google Scholar]
- 31.Kelly CR, Kunde SS, Khoruts A. Guidance on preparing an Investigational New Drug application for fecal microbiota transplantation studies. Clin Gastroenterol Hepatol. 2014;12:283–8. doi: 10.1016/j.cgh.2013.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.