Abstract
STUDY QUESTION
Does triiodothyronine (T3) regulate the secretion of angiogenic growth factors and cytokines by human decidual cells isolated from early pregnancy?
SUMMARY ANSWER
T3 modulates the secretion of specific angiogenic growth factors and cytokines, with different regulatory patterns observed amongst various isolated subpopulations of human decidual cells and with a distinct change between the first and second trimesters of pregnancy.
WHAT IS KNOWN ALREADY
Maternal thyroid dysfunction during early pregnancy is associated with complications of malplacentation including miscarriage and pre-eclampsia. T3 regulates the proliferation and apoptosis of fetal-derived trophoblasts, as well as promotes the invasive capability of extravillous trophoblasts (EVT). We hypothesize that T3 may also have a direct impact on human maternal-derived decidual cells, which are known to exert paracrine regulation upon trophoblast behaviour and vascular development at the uteroplacental interface.
STUDY DESIGN, SIZE, DURATION
This laboratory-based study used human decidua from first (8–11 weeks; n = 18) and second (12–16 weeks; n = 12) trimester surgical terminations of apparently uncomplicated pregnancies.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Primary cultures of total decidual cells, and immunomagnetic bead-isolated populations of stromal-enriched (CD10+) and stromal-depleted (CD10−) cells, uterine natural killer cells (uNK cells; CD56+) and macrophages (CD14+) were assessed for thyroid hormone receptors and transporters by immunocytochemistry. Each cell population was treated with T3 (0, 1, 10, 100 nM) and assessments were made of cell viability (MTT assay) and angiogenic growth factor and cytokine secretion (immunomediated assay). The effect of decidual cell-conditioned media on EVT invasion through Matrigel® was evaluated.
MAIN RESULTS AND THE ROLE OF CHANCE
Immunocytochemistry showed the expression of thyroid hormone transporters (MCT8, MCT10) and receptors (TRα1, TRβ1) required for thyroid hormone-responsiveness in uNK cells and macrophages from the first trimester. The viability of total decidual cells and the different cell isolates were unaffected by T3 so changes in cell numbers could not account for any observed effects. In the first trimester, T3 decreased VEGF-A secretion by total decidual cells (P < 0.05) and increased angiopoietin-2 secretion by stromal-depleted cells (P < 0.05) but in the second trimester total decidual cells showed only increased angiogenin secretion (P < 0.05). In the first trimester, T3 reduced IL-10 secretion by total decidual cells (P < 0.05), and reduced granulocyte macrophage colony stimulating factor (P < 0.01), IL-8 (P < 0.05), IL-10 (P < 0.01), IL-1β (P < 0.05) and monocyte chemotactic protein -1 (P < 0.001) secretion by macrophages, but increased tumour necrosis factor-α secretion by stromal-depleted cells (P < 0.05) and increased IL-6 by uNK cells (P < 0.05). In contrast, in the second trimester T3 increased IL-10 secretion by total decidual cells (P < 0.01) but did not affect cytokine secretion by uNK cells and macrophages. Conditioned media from first trimester T3-treated total decidual cells and macrophages did not alter EVT invasion compared with untreated controls. Thus, treatment of decidual cells with T3 resulted in changes in both angiogenic growth factor and cytokine secretion in a cell type-specific and gestational age-dependent manner, with first trimester decidual macrophages being the most responsive to T3 treatment, but these changes in decidual cell secretome did not affect EVT invasion in vitro.
LIMITATIONS, REASONS FOR CAUTION
Our results are based on in vitro findings and we cannot be certain if a similar response occurs in human pregnancy in vivo.
WIDER IMPLICATIONS OF THE FINDINGS
Optimal maternal thyroid hormone concentrations could play a critical role in maintaining a balanced inflammatory response in early pregnancy to prevent fetal immune rejection and promote normal placental development through the regulation of the secretion of critical cytokines and angiogenic growth factors by human decidual cells. Our data suggest that there is an ontogenically determined regulatory ‘switch’ in T3 responsiveness between the first and second trimesters, and support the notion that the timely and early correction of maternal thyroid dysfunction is critical in influencing pregnancy outcomes.
STUDY FUNDING/COMPETING INTEREST(S)
This study is funded by Wellbeing of Women (RG/1082/09 to S.Y.C., M.D.K., J.A.F., L.S.L., G.E.L.) and Action Medical Research – Henry Smith Charity (SP4335 to M.D.K., S.Y.C., L.S.L., J.A.F.). The authors have no conflicts of interest to disclose.
Keywords: decidua, triiodothyronine, cytokines, angiogenic growth factors, extravillous trophoblast
Introduction
Both maternal hypothyroidism and hyperthyroidism have been associated with a range of malplacentation disorders such as miscarriage, stillbirth, prematurity, pre-eclampsia and fetal growth restriction (Krassas et al., 2010), leading to increased perinatal morbidity and mortality. Even minor perturbations in maternal thyroid activity have been linked to miscarriage (Negro et al., 2010), preterm delivery and placental abruption (Casey et al., 2005). Increasing delay in achieving euthyroidism in pregnancies complicated by maternal thyroid dysfunction has been correlated with greater obstetric risks (Abalovich et al., 2002; LaFranchi et al., 2005), suggesting that key thyroid-responsive events during fetoplacental development are gestational age-dependent and may not be corrected at a later stage of pregnancy. It is widely accepted that inadequate transplacental passage of thyroid hormone (TH) from the mother to the fetus in early pregnancy can affect fetal central nervous system development (de Escobar et al., 2008). In addition, it is postulated that maternal TH has direct effects upon placental development itself and may, thus, influence pregnancy outcome (Kilby et al., 2005).
Malplacentation disorders are characterized by shallow interstitial invasion of fetal-derived trophoblast cells into maternal decidua and myometrium, and impaired endovascular trophoblast invasion resulting in incomplete maternal spiral artery remodelling. Spiral artery remodelling is required for the establishment of high-flow, low-resistance vessels for effective maternal–fetal exchange (Khong et al., 1986). The differentiation of cytotrophoblasts from a proliferative pluripotent phenotype to extravillous trophoblasts (EVT) with an invasive phenotype starts in the first trimester and peaks in the early second trimester, with invasion completed by 20 weeks of gestation. Trophoblast differentiation, invasion, vascular remodelling and angiogenesis are tightly orchestrated by a balance of autocrine and paracrine factors, which promote or inhibit these processes. They involve the regulation of cytokine and growth factor secretions in the crosstalk between fetal-derived cells and the various maternal-derived decidual cell types (Bischof and Irminger-Finger, 2005; Moffett and Loke, 2006). Decidual leukocytes (comprised of 70% uterine natural killer (uNK) cells (Bulmer and Lash, 2005) and 20–25% macrophages (Nagamatsu and Schust, 2010)) are the major sources of these secreted factors in decidua. In addition, this complex cytokine network at the maternal–fetal interface is required to assert immune privilege to prevent immune rejection of the fetus (Challis et al., 2009). Thus, defective trophoblast invasion and aberrant expression of various cytokines and growth factors have been reported in miscarriage, preterm labour, pre-eclampsia and fetal growth restriction (Moffett and Loke, 2006; Challis et al., 2009).
The main active ligand of TH is triiodothyronine (T3). Like the human fetal-derived villous placenta and EVT (Kilby et al., 1998; Barber et al., 2005), the human maternal decidua could be TH-responsive from early pregnancy, as evidenced by the expression of the full complement of proteins that are required to mediate TH action. There are TH plasma membrane transporters monocarboxylate transporter (MCT) 8 and MCT10 in decidual stroma (Chan et al., 2006; Loubiere et al., 2010) which could facilitate cellular uptake of TH. There is generalized expression of the pre-receptor regulator deiodinase enzyme type 3 [D3; inactivates thyroxine (T4) and T3] indicating the potential to modulate local T3 concentrations (Huang et al., 2003). Decidual stroma, leukocytes and endothelium express TH receptor isoforms (TRα1, TRβ1), nuclear transcription factors that can bind to T3, to regulate transcription of TH-responsive genes (Barber et al., 2005). This indicates that decidual cells can directly respond to TH at a time before and during EVT invasion.
The increased risk of malplacentation disorders with abnormal maternal thyroid function tests during early pregnancy suggests a role for TH in events of placentation during the first and second trimesters. Indeed, in fetal-derived first trimester primary trophoblasts and choriocarcinoma cell lines, T3 has been shown to promote proliferation (Matsuo et al., 1993), cell motility (Barber et al., 2005), invasion (Oki et al., 2004; Vasilopoulou et al., 2013) and epidermal growth factor (EGF) production (Matsuo et al., 1993) as well as to suppress apoptosis (Laoag-Fernandez et al., 2004) in vitro. However, the actions of TH on the maternal-derived decidual cell types are unknown.
We hypothesize that TH plays an important role in the regulation of the cytokine and angiogenic growth factor milieu within the decidua, which could impact upon placental development and, ultimately, pregnancy outcome. In this study, we aimed to isolate the effects of T3 on specific decidual cell types in the first and second trimesters. We have: (i) confirmed that different decidual cell populations demonstrate the simultaneous expression of proteins required to respond to T3, (ii) assessed the effects of T3 upon decidual cell viability as well as angiogenic growth factor and cytokine secretion by the sum of all decidual cells, stromal-enriched and stromal-depleted cell populations, isolated uNK cells and macrophages and (iii) investigated the paracrine impact on primary EVT invasion through the use of conditioned media from T3-treated decidual cells.
Materials and Methods
Ethical approval
Human samples were collected with informed written consent and with the approval of the South Birmingham Research Ethics committee (Reference: 06/Q2707/12) and the Research and Development office of the Walsall Manor Hospitals NHS Trust (Project code: 2007013OG(W); approval number: 11070745).
Sample collection
Placental and decidual samples were obtained from women undergoing elective surgical termination of apparently uncomplicated pregnancies. Samples were collected from 8–11 weeks of gestation (n = 18; first trimester) and 12–16 weeks of gestation (n = 12; second trimester) as determined by ultrasound measurement of crown rump length or biparietal diameter prior to pregnancy termination. The fetuses were not known to have abnormal karyotypes and none of the pregnancies was complicated by thyroid disorders.
Decidua cell isolation and culture
Following collection, placental and decidual tissues were washed three times with PBS to remove excess blood. Decidual tissue was finely minced and added to 15 ml RPMI media (Invitrogen, Paisley, UK) containing 0.029% (w/v) l-Glutamine (Life Sciences), 1000 units Penicillin and Streptomycin (Life Sciences), 15 mg collagenase 1A (Sigma-Aldrich, Dorset, UK) and 585 units DNAse I (Sigma-Aldrich). The tissue was allowed to digest for 40 min at room temperature on a rocking platform. The supernatant was sieved through a 40 µm cell strainer and the cells (total decidual cells) were collected by centrifugation. The digestion step was repeated with the remaining tissue.
Total decidual cells were cultured in RPMI media supplemented with l-Glutamine (Life Sciences), Penicillin and Streptomycin (Life Sciences) and 10% (v/v) charcoal-stripped fetal calf serum (which is devoid of thyroid hormones and growth factors; FirstLink, Birmingham, UK) at a density of 1 × 105 cells/well of a 96-well plate) with 0, 1, 10 or 100 nM T3 for 24 h in a 5% CO2 incubator at 37°C.
For the purification of different decidual cell types, total decidual cells were cultured in a 75 cm2 flask and allowed to adhere overnight. Stromal-enriched, stromal-depleted, uNK cells and macrophages were purified by immunomagnetic bead selection according to the manufacturer's instructions (Miltenyi Biotec, Surrey, UK). For the selection of stromal cells, all cells were collected by trypsinization and incubated with a mouse CD10 antibody (1/100, Novocastra, Newcastle Upon Tyne, UK) for 30 min at 4°C, washed and incubated with anti-mouse IgG MicroBeads (Miltenyi Biotec). CD10 positive (stromal-enriched) and CD10 negative (stromal-depleted) cells were obtained by magnetic separation. For the selection of uNK cells, the cells in suspension were collected and incubated with a mouse CD56 antibody (1/100, Coulter, High Wycombe, UK) for 1 h at 4°C, followed by anti-mouse IgG MicroBeads (Miltenyi Biotec) and purified by magnetic separation. For the selection of macrophages, the adherent cells were collected by trypsinization, incubated with CD14 MicroBeads (Miltenyi Biotec) and purified by magnetic separation. The CD14+ cell isolates were comprised predominantly of macrophages but would also include monocytes, which can later differentiate into CD14+ macrophages as well as into CD14− dendritic cells. These CD14+ isolates were referred to as macrophages in this paper. Cell purity was assessed by immunocytochemistry (Fig. 1, column 1) and was found to be over 95% for uNK cells and over 90% for macrophages and stromal cells. All purified cell types were counted and cultured in RPMI media as for the total decidual cells in 96-well plates (1 × 105 cells/well) with 0, 1, 10 or 100 nM T3 for 24 h in a 5% CO2 incubator at 37°C. Cell-free conditioned media was collected and stored at −80°C until required.
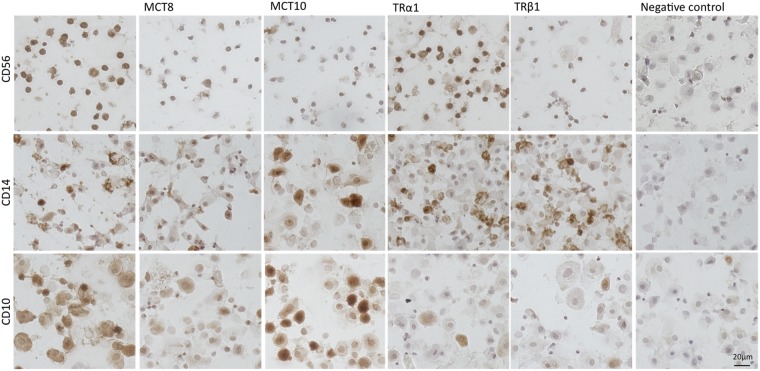
Figure 1.
Immunocytochemistry of purified decidual cell types from the first trimester demonstrating immunoreactivity for thyroid hormone transporters (monocarboxylate transporter (MCT) 8 and MCT10) and thyroid hormone receptors (TRα1 and TRβ1). Top panel: uterine natural killer (uNK) cells (CD56+), middle panel: macrophages (CD14+), bottom panel: stromal-enriched cells (CD10+). Each panel is of isolates from one representative preparation of cells. Negative controls were performed for each sample by replacing the primary antibody with non-immune serum.
Immunocytochemistry
Following purification, cells (0.5 × 105 cells per slide) were put onto microscope slides and allowed to air-dry, fixed in acetone and stored at −20°C. For immunocytochemistry, sections were washed in PBS, incubated in 2% BSA and 5% serum in PBS for 30 min to block non-specific binding and then incubated in the primary antibody for 1 h at room temperature. The antibodies used were: CD10 (1/100, Novocastra), CD56 (1/100, Novocastra), CD14 (1/100, Leica Biosystems, Newcastle Upon Tyne, UK), MCT8 [ab4790, 1/800 (44)], MCT10 [Ab2198, 1/1000 (Vasilopoulou et al., 2010)], TRα1 (1/200, Thermo Scientific, Rockford, IL, USA) and TRβ1 (1/200, Thermo Scientific). The samples were further processed using an anti-mouse or anti-rabbit avidin/biotin kit (Vectastain Elite, Vector Laboratories, Peterborough, UK) as per the manufacturer's instructions. The staining was developed using a DAB Substrate kit (Vector Laboratories). The samples were dehydrated in a series of graded ethanols, cleared in histoclear and mounted. Negative controls were performed in parallel using non-immune serum instead of the primary antibody. The TRα2 isoform, which does not bind T3 (Koenig et al., 1989), and TRβ2, which is not expressed in human placenta (Kilby et al., 1998), were not evaluated. Immunocytochemistry was performed in first trimester decidual cell isolates but not performed in second trimester isolates due to limited sample availability.
Cell viability assay
Cell viability was assessed 24 h after T3 treatment (0, 1, 10 or 100 nM) using the methyltetrazoleum (MTT; Sigma-Aldrich) colourimetric assay as described previously (Barber et al., 2005). Within each experiment, each condition was assessed in four replicates and the absorbance values were normalized to the values obtained with no T3 treatment (0 nM).
Assessment of angiogenic growth factor and cytokine secretion
The secretion of angiogenic growth factors and cytokines in conditioned media (collected 24 h after T3 treatment) was assessed using FAST Quant human angiogenesis array and human cytokine II array according to the manufacturer's instructions (Whatman GE Healthcare, Sanford, ME, USA) as previously described (Lash et al., 2010; Naruse et al., 2010). The angiogenesis array included angiogenin, angiopoietin-2 (Ang-2), fibroblast growth factor basic (FGF-b), tissue inhibitor of metalloproteinase I (TIMP-1), intracellular adhesion molecule 1 (ICAM-1), vascular endothelial growth factor-A (VEGF-A), platelet-derived growth factor-BB (PDGF-BB) and keratinocyte growth factor (KGF). The multi-cytokine panel tested for interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL12p70, granulocyte macrophage colony stimulating factor (GM-CSF), monocyte chemotactic protein-1 (MCP-1) and RANTES (regulated on activation normal T cell expressed and secreted). Interferon (IFN)-γ and tumour necrosis factor (TNF)-α were quantified by ELISA (Duoset Development System, R&D Systems, Abingdon, UK) according to the manufacturer's instructions.
The concentrations of some factors in the conditioned media were below or above the concentration range covered by the manufacturer's standard curve. Where one or more of the T3 treatments resulted in concentrations outside the standard curve limits for a particular factor, the sample was excluded from further analysis. Only factors where at least three cell preparations had a full dataset across the T3 concentrations were included. The final number of preparations included in the analysis for each cell population and each secretory factor are shown in Supplementary data, Tables SI and SII. The results were normalized to the values obtained with no T3 treatment (0 nM) within each sample.
EVT isolation and invasion assay
Primary EVT cells were isolated from first trimester human placentae, as described previously (Lash et al., 2010) and cultured in cell culture inserts (8 µm membrane pore size) coated with 10 µl of growth factor-reduced Matrigel® matrix (BD Biosciences, Erembodegem, Belgium). Below the inserts, conditioned media from cultured total decidual cells or macrophages (from 8 to 11 weeks of gestation samples) was added at a dilution of 1 in 3 in normal culture medium (DMEM:F12). As previous studies have reported peak EVT invasion with T3 treatment at 10 nM in vitro (Oki et al., 2004; Vasilopoulou et al., 2013), we used conditioned media from decidual cells treated with 10 nM T3 compared with that treated with 0 nM. Since T3 per se can promote EVT invasion, as controls we also collected unconditioned media (supplemented with 0 or 10 nM T3) from cell-free wells alongside each experiment to account for the natural degradation of T3 and the decline in T3 activity over the culture period and the freeze-thaw cycle. This enabled us to distinguish between the effects of residual T3 in the media and the effects of the decidual cell secretome. Cell invasion through Matrigel® was assessed 60 h after culture by counting all the invaded cells visualized with Mayer's haematoxylin and eosin (H&E) staining. The invasion index was determined and expressed as the ratio of invaded cells in the experimental group relative to that in the control group (cell-free media, 0 nM T3).
Statistical analysis
Data were analysed using the Minitab® statistical software (version 15). Repeated measures analysis of variance (ANOVA) was performed using the general linear model. For the angiogenic growth factor and cytokine data, each cell population in each trimester was considered separately with T3 dose and factor-type as variables. For the EVT invasion assay, T3 dose and cell-conditioning were the variables. Bonferroni pairwise multiple comparison (with control 0 nM T3) post hoc tests were used to assess differences between individual groups. Residuals for all data sets passed the normality test as determined using the Kolmogorov–Smirnov test. Statistical significance was taken as P < 0.05.
Results
Expression of TH transporters and receptors in decidual cells
The simultaneous protein expression of the TH transporters, MCT8 and MCT10, and TH receptors, TRα1 and TRβ1, was demonstrated by immunocytochemistry in uNK cells (CD56+) and macrophages (CD14+) isolated from the first trimester decidua (Fig. 1) confirming previous immunohistochemistry findings using paraffin-embedded intact human decidual sections (Barber et al., 2005; Chan et al., 2006; Loubiere et al., 2010). This indicates the capability of these isolated cells to respond to TH. As shown in Fig. 1, uNK cells showed ubiquitous TRα1 expression and many of the cells are also positive for TRβ1, MCT8 and MCT10. Isolated macrophages demonstrated widespread immunoreactivity for both MCT8 and MCT10 as well as for both TRα1 and TRβ1. On the other hand, decidual stromal-enriched cells (CD10+) showed little immunopositivity for the TH receptors, even though many were immunopositive for MCT8 and MCT10, as previously demonstrated by immunohistochemistry in intact human decidual tissue (Loubiere et al., 2010).
Effect of T3 on decidual cell viability as measured by MTT assay
The viability of total decidual cells, stromal-enriched and stromal-depleted cells, and isolates of uNK cells and macrophages from the first (Supplementary data, Figure S1) and second (data not shown) trimesters were unaffected by T3 treatment. Thus, any changes in angiogenic growth factor or cytokine concentrations in conditioned media in these subsequent experiments is not a consequence of altered cell numbers but a true reflection of T3-responsive regulation of their secretion.
Effect of T3 on angiogenic growth factor secretion by decidual cells
To investigate TH action in maternal decidua, we assessed the secretory responses of decidual cells cultured in vitro to treatment by the active TH ligand, T3. We first examined angiogenic growth factor secretion.
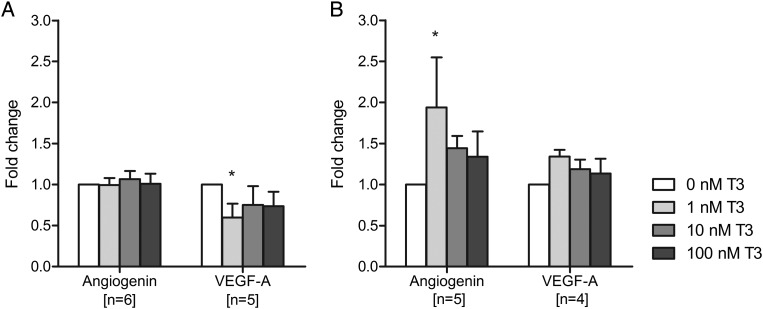
In total decidual cells from first trimester samples, T3 treatment decreased VEGF-A secretion, with the greatest effect at a dose of 1 nM (40% reduction compared with no T3; P < 0.05; Fig. 2A). In the second trimester, total decidual cells demonstrated increased angiogenin secretion in response to T3 treatment with the greatest effect observed with 1 nM T3 compared with no T3 (1.9-fold increase, P < 0.05; Fig. 2B). However, the T3 effect on VEGF-A secretion was lost. There were no significant T3 effects on Ang-2, FGF-basic and ICAM-1 secretion by first and second trimester total decidual cells (Supplementary data, Table SI).
Figure 2.
Effect of triiodothyronine (T3) on angiogenin and VEGF-A (vascular endothelial growth factor-A) secretion by total decidual cells after 24 h of treatment with 0, 1, 10 or 100 nM T3. (A) Isolated from first trimester samples, (B) isolated from second trimester samples. Within each experiment, the angiogenic growth factor concentration was compared with that after no T3 treatment (0 nM), which was given an arbitrary value of 1. Bars represent mean + SEM. Statistical significance *P < 0.05.
We then assessed the effects on stromal-depleted cells, which comprise of the major growth factor secretors such as leukocytes, as well as endothelium and epithelial cells. Treatment of first trimester stromal-depleted cells with 1 nM T3 resulted in a 2.7-fold increase in the secretion of Ang-2 compared with no T3 (P < 0.05; Table I). In contrast, first trimester stromal-enriched cells did not demonstrate any significant T3-responsive effects.
Table I.
Effect of T3 on selected angiogenic growth factor secretion by first trimester stromal-depleted (CD10−) and stromal-enriched (CD10+) cells
| T3 (nM) | Angiogenin | Ang-2 | VEGF-A | |
|---|---|---|---|---|
| CD10− | N = 5 | N = 4 | N = 5 | |
| 0 | 1 | 1 | 1 | |
| 1 | 1.1 ± 0.2 | 2.7 ± 1.3* | 1.8 ± 0.8 | |
| 10 | 1.1 ± 0.3 | 1.2 ± 0.1 | 1.8 ± 0.8 | |
| 100 | 1.2 ± 0.4 | 0.9 ± 0.1 | 1.6 ± 0.7 | |
| CD10+ | N = 6 | Above limit | N = 6 | |
| 0 | 1 | 1 | ||
| 1 | 1.1 ± 0.1 | 1.0 ± 0.1 | ||
| 10 | 1.1 ± 0.1 | 1.0 ± 0.1 | ||
| 100 | 1.2 ± 0.2 | 1.2 ± 0.2 |
Data are normalized to 0 nM within each experiment and presented as mean ± SEM.
Ang-2 concentrations in CD10+ cells and fibroblast growth factor basic (FGF-basic), tissue inhibitor of metalloproteinase I (TIMP-1), intracellular adhesion molecule 1 (ICAM-1) concentrations in both CD10− and CD10+ cells were above the manufacturer's standard curve limit. Platelet-derived growth factor-BB (PDGF-BB) was below the detection limit in all samples and keratinocyte growth factor (KGF) was only detectable in two or less samples per experimental set.
Ang-2, Angiopoietin-2; VEGF-A, vascular endothelial growth factor-A.
*P<0.05 compared with no T3 treatment (0 nM).
Isolated uNK cells from the first trimester showed no significant T3-responsive effects (Fig. 3A), suggesting that other cell types are responsible for the increased Ang-2 secretion observed in the stromal-depleted cell population. These may include secretion by macrophages, where Ang-2 concentrations were above the upper limit of the standard curve. Unlike the first trimester, second trimester uNK cells demonstrated trends of dose-dependent increases in the secretion of angiogenin, Ang-2, TIMP-1, ICAM-1 and VEGF-A, which just failed to reach statistical significance (overall effect of T3: P = 0.077; Fig. 3B).
Figure 3.
Effect of triiodothyronine (T3) on angiogenic growth factor secretion by uterine natural killer (uNK) cells after 24 h of treatment with 0, 1, 10 or 100 nM T3. (A) Isolated from first trimester samples, (B) isolated from second trimester samples. Within each experiment, the angiogenic growth factor concentration was compared with that after no T3 treatment (0 nM), which was given an arbitrary value of 1. Bars represent mean + SEM. FGF, fibroblast growth factor; TIMP-1, tissue inhibitor of metalloproteinase I; ICAM-1, intracellular adhesion molecule 1; VEGF-A, vascular endothelial growth factor-A.
Of the angiogenic growth factors secreted by macrophages which were within the detection thresholds (Angiogenin, VEGF-A, FGF-basic), none demonstrated statistically significant changes in response to T3 treatment (Supplementary data, Table SI).
PDGF-BB was below the detection limit in all decidual samples and KGF was only just detectable in a few samples. TIMP1 concentrations by total decidual cells and macrophages were above the limit of the standard curve.
Effect of T3 on cytokine secretion by decidual cells
There were substantial changes in the secretion of cytokines by decidual cells following T3 treatment in vitro, predominantly in the first trimester.
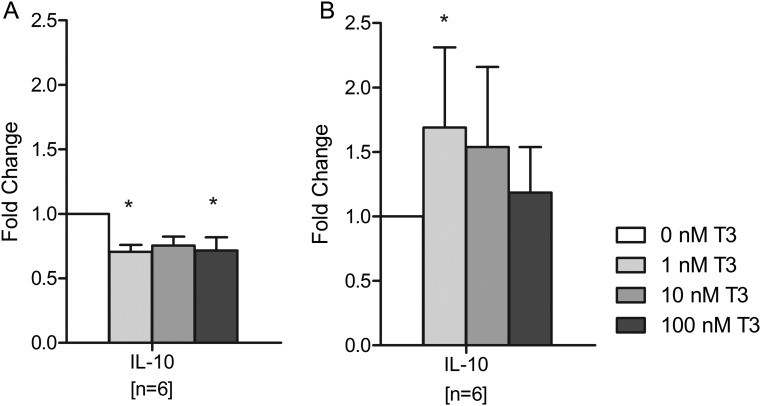
In first trimester total decidual cells, T3 treatment significantly decreased IL-10 secretion with the most prominent effect observed when cells were treated with 1 or 100 nM T3 (30% reduction; P < 0.05 for both; Fig. 4A). In contrast, IL-10 secretion by second trimester total decidual cells was significantly increased in response to T3, with the highest effect occurring at 1 nM (70% increase; P < 0.05; Fig. 4B). In both first and second trimester total decidual cells, T3 treatment did not result in any significant differences in IL-8, GMCSF, IL-1β, MCP-1, IFN-γ and TNF-α secretion (Supplementary data, Table SII).
Figure 4.
Effect of triiodothyronine (T3) on interleukin (IL)-10 secretion by total decidual cells after 24 h of treatment with 0, 1, 10 or 100 nM T3. (A) Isolated from first trimester samples, (B) isolated from second trimester samples. Within each experiment, the IL-10 concentration was compared with that after no T3 treatment (0 nM), which was given an arbitrary value of 1. Bars represent mean + SEM. Statistical significance *P < 0.05.
In stromal-depleted cells from the first trimester (Table II), the secretion of MCP-1 was down-regulated by T3, particularly at 10 nM which caused a 30% reduction (P < 0.05), but TNF-α secretion was up-regulated by 30% at 1 and 10 nM T3 compared with no T3 (P < 0.05 for both). No T3-responsive effects were observed in stromal-enriched cells.
Table II.
Effect of T3 on selected cytokine secretion by first trimester stromal-depleted (CD10−) and stromal-enriched (CD10+) cells
| T3 (nM) | IL-10 | MCP-1 | TNF-α | |
|---|---|---|---|---|
| CD10− | N = 6 | N = 6 | N = 6 | |
| 0 | 1 | 1 | 1 | |
| 1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.3 ± 0.2* | |
| 10 | 0.7 ± 0.1 | 0.7 ± 0.1* | 1.3 ± 0.2* | |
| 100 | 0.7 ± 0.1 | 0.8 ± 0.1 | 1.3 ± 0.2 | |
| CD10+ | N = 5 | N = 6 | N = 7 | |
| 0 | 1 | 1 | 1 | |
| 1 | 1.3 ± 0.2 | 0.9 ± 0.1 | 0.9 ± 0.1 | |
| 10 | 1.1 ± 0.3 | 1.0 ± 0.1 | 0.9 ± 0.1 | |
| 100 | 1.2 ± 0.2 | 1.2 ± 0.1 | 0.7 ± 0.1 |
Data are normalized to 0 nM within each experiment and presented as mean ± SEM.
There were no significant changes in IL-8, GM-CSF (granulocyte macrophage colony stimulating factor), IL-1β and interferon-γ in response to T3 treatment (data not shown). RANTES (regulated on activation normal T cell expressed and secreted) and interleukin (IL)-6 concentrations in both CD10− and CD10+ cells were above the manufacturer's standard curve limit. IL12p70 and IL-4 were below the detection limit in all samples and IL-2 was only detectable in two or less samples per experimental set.
IL, interleukin; MCP, monocyte chemotactic protein; TNF, tumour necrosis factor.
*P < 0.05 compared with no T3 treatment (0 nM).
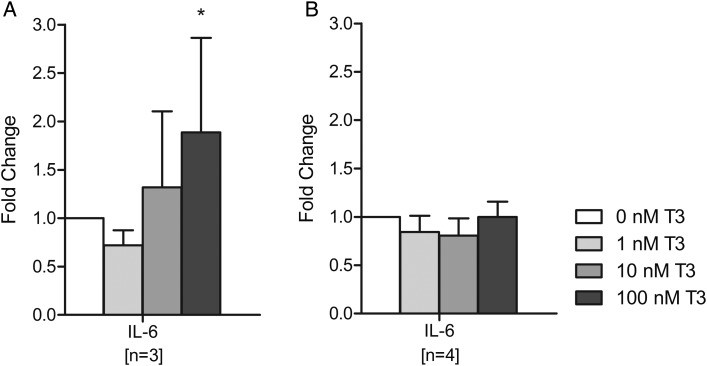
In the first trimester, treatment of uNK cells only with the highest dose of T3 (100 nM) resulted in a statistically significant increase in IL-6 secretion (1.8-fold compared with no T3; P < 0.05; Fig. 5A). However, T3 treatment had no significant effects on IL-6 secretion by second trimester uNK cells (Fig. 5B). The secretion of other quantifiable cytokines by uNK cells did not demonstrate any significant changes in response to T3 treatment (Supplementary data, Table SII).
Figure 5.
Effect of triiodothyronine (T3) on interleukin (IL)-6 secretion by uterine natural killer (uNK) cells after 24 h of treatment with 0, 1, 10 or 100 nM T3. (A) Isolated from first trimester samples, (B) isolated from second trimester samples. Within each experiment, the IL-6 concentration was compared with that after no T3 treatment (0 nM), which was given an arbitrary value of 1. Bars represent mean + SEM. Statistical significance *P < 0.05.
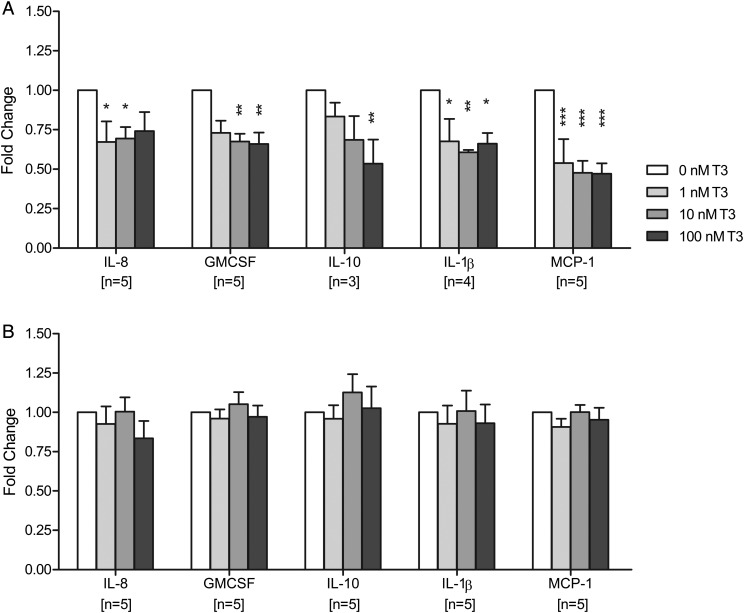
The cells that were most affected by T3 treatment were the first trimester decidual macrophages, where the secretion of multiple cytokines was decreased in response to T3 treatment (Fig. 6A), with the exception of IFN-γ and TNF-α which both showed no significant changes (Supplementary data, Table SII). The secretion of IL-8 was reduced by 30% following treatment with 1 or 10 nM T3 compared with no T3 (P < 0.05 for both) and the secretion of GM-CSF was decreased by 35% with 10 or 100 nM T3 compared with no T3 (P < 0.01 for both). IL-10 secretion was also reduced with T3 treatment with the most pronounced effect when the cells were treated with 100 nM T3 (50% reduction compared with no T3; P < 0.01), suggesting that this effect may account for the overall IL-10 reduction observed in total decidual cells. Furthermore, IL-1β secretion was reduced by 30% at 1 nM T3 (P < 0.05), 40% at 10 nM T3 (P < 0.01) and 35% at 100 nM T3 (P < 0.05). Finally, MCP-1 secretion was most notably affected by T3 treatment with a substantial 50% reduction observed at all T3 doses (P < 0.001). In contrast, macrophages from second trimester decidua demonstrated no significant changes in response to T3 treatment (Fig. 6B).
Figure 6.
Effect of triiodothyronine (T3) on cytokine secretion by decidual macrophages after 24 h of treatment with 0, 1, 10 or 100 nM T3. (A) Isolated from first trimester samples, (B) isolated from second trimester samples. Within each experiment, the cytokine concentration was compared with that after no T3 treatment (0 nM), which was given an arbitrary value of 1. Bars represent mean + SEM. Statistical significance *P < 0.05, **P < 0.01, ***P < 0.001. IL, interleukin; GM-CSF, granulocyte macrophage colony stimulating factor; MCP-1, monocyte chemotactic protein-1.
In all decidual samples, IL12p70 and IL-4 were below the detection limit whilst IL-2 was only detectable at low concentrations in a handful of samples. RANTES and IL-6 concentrations were above the upper limit of the standard curve in conditioned media from total decidual cells and macrophages.
Effect of conditioned media from T3-treated decidual cells on EVT invasion
Given our observations that T3 treatment affects angiogenic growth factors and cytokine secretion by decidual cells, we next investigated whether the changes in the decidual cell secretome could regulate EVT invasion in a paracrine manner. Since the effects of T3 were more prominent in first trimester cells and particularly so in first trimester macrophages, we investigated this using conditioned media from first trimester total decidual cells and first trimester decidual macrophages.
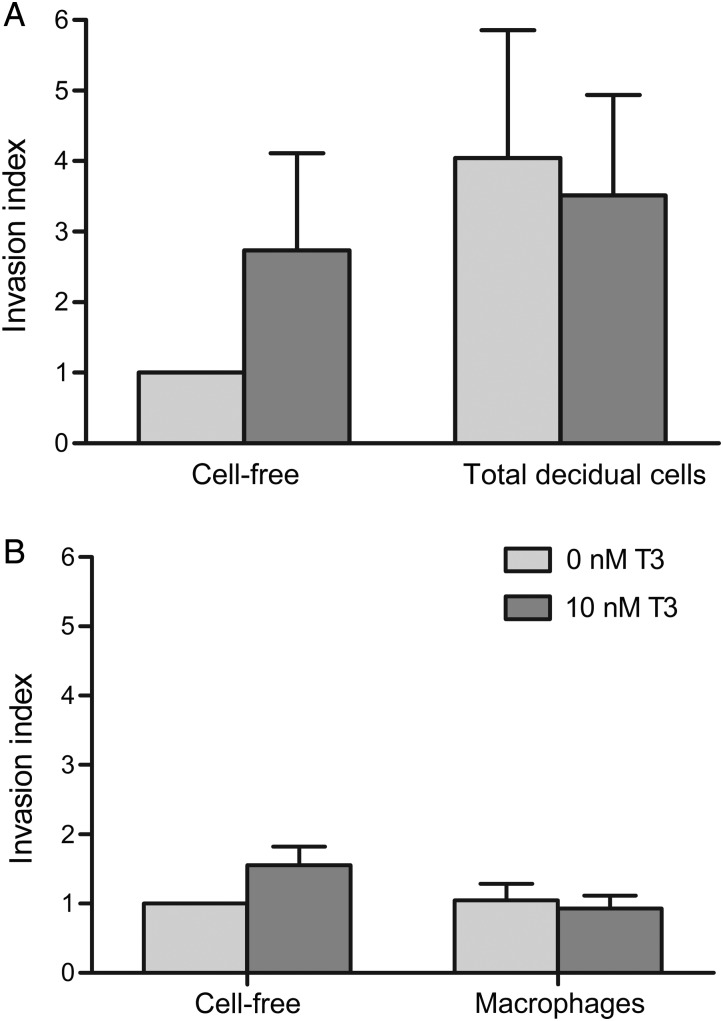
When we compared the invasiveness of first trimester EVT cells treated with conditioned media obtained from T3-treated total decidual cells (Fig. 7A) or from T3-treated macrophages (Fig. 7B) with their respective untreated cellular controls, we found no significant differences. Since T3 is known to have a direct pro-invasive effect upon EVT, it was important to determine if the initial T3 added to media could still affect EVT invasion directly, and to disentangle this effect from that of the decidual secretome. Results from cell-free media showed that T3 added to wells in initial experiments no longer had a statistically significant effect in subsequent EVT invasion experiments, although a trend towards increased invasive capacity was still seen. A similar decline in T3 effectiveness due to its natural degradation in decidual cell-conditioned media from parallel experiments is assumed. In fact, it is likely that there would be even less residual T3 in the decidual cell-conditioned media due to T3 inactivation by cellular D3 activity, which is not present in cell-free wells. Thus, we conclude that the effect of decidual cell-conditioned media on EVT invasion is largely independent of the initial T3 added.
Figure 7.
Effect of conditioned media from triiodothyronine (T3)-treated first trimester total decidual cells (A) or T3-treated first trimester macrophages (B) on invasion by first trimester extravillous trophoblasts (EVT). Unconditioned media (supplemented with 0 or 10 nM T3) from cell-free wells alongside each experiment were used as controls (Cell-free). Within each experiment, each condition was performed in duplicate and the results normalized to the mean value for the control (0 nM T3, cell-free) which was given an arbitrary value of 1. Bars represent mean of nine experiments + SEM.
When we compared the invasiveness of EVT treated with conditioned media from total decidual cells with media collected from cell-free wells, there appeared to be an overall trend towards increased invasion although this did not reach statistical significance (P = 0.3; Fig. 7A). The conditioned media from decidual macrophages also did not alter EVT invasiveness when compared with media collected from cell-free wells (P = 0.2; Fig. 7B). These observations indicate that the summation of secretome effects from either total decidual cells or macrophages did not affect EVT invasion in our experimental model.
Discussion
These results demonstrate that specific maternal-derived decidual cells show a coordinated expression of the proteins required for TH action and are T3-responsive in early human gestation. The effects of T3 on angiogenic growth factor and cytokine secretion in vitro are cell type-specific and gestational age-dependent. Opposing effects of T3 between the first and second trimester on the secretion of IL-10, one of the central regulatory cytokines in human pregnancy (Thaxton and Sharma, 2010; Brogin et al., 2012), indicate an ontogenically-determined regulatory ‘switch’ in T3 responsiveness within human decidua. Furthermore, first trimester macrophages demonstrated the most marked T3 responsiveness indicated by the down-regulation of a range of cytokines, a response that was attenuated in second trimester macrophages. Similarly, in first trimester uNK cells, T3 invoked different profiles of angiogenic growth factor and cytokine secretion compared with second trimester uNK cells. Overall, T3 appears to participate in the timely regulation of specific paracrine factors in the human decidua, which could facilitate placentation events appropriate for the stage of gestation.
The stromal-enriched cells which lacked TR expression showed no change in angiogenic growth factor or cytokine secretion with T3 treatment. However, their expression of MCT8 and MCT10 could facilitate TH uptake and these cells could play a role in regulating local tissue TH concentrations through deiodinase metabolism (Huang et al., 2003).
Circulating total T3 concentrations in adults are in the region of 0.1–1 nM but the local intracellular concentrations of T3 in decidua are not known and may be different as a result of local deiodinase type 2 (converts T4 to T3) and type 3 (inactivates T3) activities. Almost all of the statistically significant decidual cell responses in our study occurred at a physiological concentration of 1 nM T3 treatment in vitro, with many also occurring at higher T3 concentrations.
Angiogenic growth factors are critical to the development of both maternal and fetal vasculature at the uteroplacental interface. Our data suggest that T3 affects the timely decidual secretion of three key angiogenic growth factors implicated in human placentation. In the first trimester, VEGF-A, which promotes maternal and fetoplacental angiogenesis (Wulff et al., 2003) as well as EVT motility (Lash et al., 1999), is down-regulated by T3 in total decidual cells. This is appropriate as it is at a time prior to the peak of EVT invasion when invasion needs to be tightly controlled. In contrast, Ang-2, which destabilizes vasculature and mediates some of the initial stages of spiral artery remodelling (Robson et al., 2012), is appropriately up-regulated in stromal-depleted cells in preparation for the next phase of placentation. In the second trimester, the secretion of angiogenin (a potent inducer of angiogenesis whose placental expression increases with gestation (Rajashekhar et al., 2002)) by total decidual cells is suitably up-regulated by T3.
In normal pregnancy, the balance of the anti-inflammatory versus pro-inflammatory milieu changes in favour of immunosuppression to prevent immune rejection of the fetal allograft and promote normal placental cytoarchitectural development (Moffett and Loke, 2006; Challis et al., 2009). Our data support a role for T3 in the regulation of this balance at the uteroplacental interface.
Central to this T3-modulated response is the regulation of decidual IL-10 secretion. The anti-inflammatory response of pregnancy is dominated by a significant rise in IL-10 during the first trimester. A high level of IL-10 is sustained across the rest of pregnancy before its decline prior to the onset of labour (Thaxton and Sharma, 2010). In addition, IL-10 promotes EVT differentiation (Moreau et al., 1999), inhibits EVT invasion (Pang et al., 2008) and indirectly stimulates angiogenesis (Thaxton and Sharma, 2010). Our results indicate that T3 down-regulates IL-10 secretion by first trimester total decidual cells and macrophages. This is in contrast to the up-regulation of IL-10 by second trimester total decidual cells, an effect mediated through cells other than uNK cells and macrophages. T3 appears to modulate and prevent excessive IL-10 production initially, perhaps to control EVT differentiation, but at the peak of EVT invasion and spiral artery remodelling in the early second trimester T3 increases IL-10 in the decidual secretome. T3 may play an important role in maintaining a high level of IL-10 from the second trimester of pregnancy, which is a feature associated with a reduced risk of pre-eclampsia, fetal growth restriction and preterm birth (Thaxton and Sharma, 2010; Brogin et al., 2012). It is tempting to speculate that maternal thyroid dysfunction could affect pregnancy outcome through the inappropriate and untimely modulation of this key cytokine, IL-10, at the maternal–fetal interface.
T3 also appears to increase the decidual secretion of the pro-inflammatory cytokine, TNF-α by first trimester stromal-depleted cells, which could indirectly promote decidual macrophage recruitment (Lockwood et al., 2006; Renaud et al., 2009).
Amongst the decidual cell types examined, first trimester macrophages showed the most widespread expression of TH transporters and receptors and appeared to be the most T3-responsive. However, these decidual macrophages became completely unresponsive to T3 in the second trimester, suggesting the existence of a potent ontogenically determined ‘switch’ in T3 responsiveness within this cell type. In the first trimester, T3 reduced the secretion of the macrophage activator, GM-CSF [which is increased in pre-eclamptic decidua (Huang et al., 2010)], and the macrophage chemoattractant, MCP-1. This suggests that T3 could be participating in the autoregulation of macrophage numbers and responses within the decidua to prevent excessive macrophage recruitment which could promote EVT apoptosis (Wu et al., 2012) and impair endovascular EVT invasion (Lockwood et al., 2006) leading to complications like pre-eclampsia and fetal growth restriction. In addition, the pro-inflammatory cytokines, IL-8 and IL-1β, and the anti-inflammatory IL-10 were similarly down-regulated. The ability of a single factor to reduce the secretion of a mixture of anti- and pro-inflammatory cytokines simultaneously by the same cell type at the uteroplacental interface is not unusual and has previously been reported for other factors such as thrombopoietin (Segerer et al., 2013).
T3 has previously been shown to regulate angiogenic growth factor and cytokine secretion during bone marrow-derived dendritic cell maturation (Mascanfroni et al., 2008) and in human hepatocarcinoma cells (Bockhorn et al., 2007). The mechanisms underlying the T3 regulation of decidual cell secretome remain to be determined. One possibility is that T3 regulates the transcription of these genes, either directly or indirectly, which could be substantiated by quantification of gene expression. For instance, in rat liver a T3-induced hypermetabolic state increases oxidative stress and enhances the activity of redox sensitive transcription factors, leading to increased transcription of antioxidant cytokines such as TNFα and IL-10 (Fernandez et al., 2006).
The mechanism that underlies the observed ontogenically determined ‘switch’ in T3 responsiveness in different subpopulations of decidual cells is also not known. It is possible that quantitative changes in the expression of TH transporters, receptors or deiodinase enzymes between the first and second trimesters affect the ability of the cells to take-up and respond to T3. In addition, the apparent summation of T3 effects observed in stromal-enriched and stromal-depleted cells does not always correspond with the T3 response seen in total decidual cell cultures (e.g. VEGF-A, IL-10 secretion) suggesting that interactions across the two cell populations play a role in regulating the secretion of these factors.
Amongst the secreted factors found to be regulated by T3 in decidua, TNF-α (Otun et al., 2011), GM-CSF (Wu et al., 2012) and IL-10 (Pang et al., 2008) have all been reported to attenuate EVT invasion. IL-1β and VEGF-A promote EVT motility (Lash et al., 1999; Prutsch et al., 2012) and IL-8 promotes EVT invasion (De Oliveira et al., 2010) whilst IL-6 has no effect (Champion et al., 2012). However, we did not observe the expected increase in EVT invasion in vitro as a result of T3-induced suppression of IL-10 in first trimester total decidual cell secretome, suggesting that there are other factors counteracting the lack of IL-10. Similarly, the T3-induced changes in first trimester macrophage secretome included the suppression of both pro-invasive and inhibitory factors such that the overall summation of secretome changes resulted in no effect on EVT invasion in our experimental model. None the less this result does not diminish the potential importance of the T3-induced changes in decidual secretome upon other placentation events occurring in vivo, such as immune cell recruitment and function, angiogenesis and vascular remodelling, which we have not yet investigated.
In conclusion, optimal maternal and uteroplacental TH concentrations could play a critical role in maintaining a balanced inflammatory response in pregnancy to prevent fetal immune rejection and promote normal placental development through the regulation of the secretion of critical cytokines and angiogenic growth factors by human decidual cells in a cell type-specific and gestational age-dependent manner. The nature of the ontogenically determined regulatory ‘switch’ in T3 responsiveness between the first and second trimester remains to be discovered. Our data support the notion that timely and early correction of maternal thyroid dysfunction by the first trimester of pregnancy may be critical in influencing pregnancy outcomes.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
E.V., L.S.L., G.E.L., O.O., C.J.M., J.A.F., M.D.K. and S.Y.C. made substantial contributions to the study conception and design or the acquisition of data or the analysis and interpretation of data. E.V., G.E.L., C.J.M., J.A.F., M.D.K. and S.Y.C. drafted the article or revised it critically for important intellectual content. E.V., L.S.L., G.E.L., O.O., C.J.M., J.A.F., M.D.K. and S.Y.C. gave final approval of the version to be published.
Funding
This study is funded by Wellbeing of Women (RG/1082/09 to S.Y.C., M.D.K., J.A.F., L.S.L., G.E.L.) and Action Medical Research – Henry Smith Charity (SP4335 to M.D.K., S.Y.C., L.S.L., J.A.F.). Funding to pay the Open Access publication charges for this article was provided by Wellbeing of Women, UK.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We thank Mr Merlin Walter for help with the cytokine assays.
References
- Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12:63–68. doi: 10.1089/105072502753451986. [DOI] [PubMed] [Google Scholar]
- Barber KJ, Franklyn JA, McCabe CJ, Khanim FL, Bulmer JN, Whitley GSJ, Kilby MD. The in vitro effects of triiodothyronine on epidermal growth factor-induced trophoblast function. J Clin Endocrinol Metab. 2005;90:1655–1661. doi: 10.1210/jc.2004-0785. [DOI] [PubMed] [Google Scholar]
- Bischof P, Irminger-Finger I. The human cytotrophoblastic cell, a mononuclear chameleon. Int J Biochem Cell Biol. 2005;37:1–16. doi: 10.1016/j.biocel.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Bockhorn M, Frilling A, Benko T, Best J, Sheu SY, Trippler M, Schlaak JF, Broelsch CE. Tri-iodothyronine as a stimulator of liver regeneration after partial and subtotal hepatectomy. Eur Surg Res. 2007;39:58–63. doi: 10.1159/000098443. [DOI] [PubMed] [Google Scholar]
- Brogin MJ, Cirino Ruocco AM, Vernini JM, Rudge MV, Calderon IM. Interleukin 10 and tumor necrosis factor-alpha in pregnancy: aspects of interest in clinical obstetrics. ISRN Obstet Gynecol. 2012;2012:230742. doi: 10.5402/2012/230742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol. 2005;42:511–521. doi: 10.1016/j.molimm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–245. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, III, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- Champion H, Innes BA, Robson SC, Lash GE, Bulmer JN. Effects of interleukin-6 on extravillous trophoblast invasion in early human pregnancy. Mol Hum Reprod. 2012;18:391–400. doi: 10.1093/molehr/gas010. [DOI] [PubMed] [Google Scholar]
- Chan SY, Franklyn JA, Pemberton HN, Bulmer JN, Visser TJ, McCabe CJ, Kilby D. Monocarboxylate transporter 8 expression in the human placenta: the effects of severe intrauterine growth restriction. J Endocrinol. 2006;189:465–471. doi: 10.1677/joe.1.06582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Escobar GM, Ares S, Berbel P, Obregon MJ, del Rey FE. The changing role of maternal thyroid hormone in fetal brain development. Semin Perinatol. 2008;32:380–386. doi: 10.1053/j.semperi.2008.09.002. [DOI] [PubMed] [Google Scholar]
- De Oliveira LG, Lash GE, Murray-Dunning C, Bulmer JN, Innes BA, Searle RF, Sass N, Robson SC. Role of interleukin 8 in uterine natural killer cell regulation of extravillous trophoblast cell invasion. Placenta. 2010;31:595–601. doi: 10.1016/j.placenta.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Fernandez V, Tapia G, Varela P, Romanque P, Cartier-Ugarte D, Videla LA. Thyroid hormone-induced oxidative stress in rodents and humans: a comparative view and relation to redox regulation of gene expression. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142:231–239. doi: 10.1016/j.cbpc.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab. 2003;88:1384–1388. doi: 10.1210/jc.2002-021291. [DOI] [PubMed] [Google Scholar]
- Huang SJ, Zenclussen AC, Chen CP, Basar M, Yang H, Arcuri F, Li M, Kocamaz E, Buchwalder L, Rahman M, et al. The implication of aberrant GM-CSF expression in decidual cells in the pathogenesis of preeclampsia. Am J Pathol. 2010;177:2472–2482. doi: 10.2353/ajpath.2010.091247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong TY, Dewolf F, Robertson WB, Brosens I. Inadequate Vascular-Response to Placentation in Preeclampsia (Pe) and Intrauterine Fetal Growth-Retardation (Iugr) J Pathol. 1986;148:A101. [Google Scholar]
- Kilby MD, Verhaeg J, Gittoes N, Somerset DA, Clark PM, Franklyn JA. Circulating thyroid hormone concentrations and placental thyroid hormone receptor expression in normal human pregnancy and pregnancy complicated by intrauterine growth restriction (IUGR) J Clin Endocrinol Metab. 1998;83:2964–2971. doi: 10.1210/jcem.83.8.5002. [DOI] [PubMed] [Google Scholar]
- Kilby MD, Barber K, Hobbs E, Franklyn JA. Thyroid hormone action in the placenta. Placenta. 2005;26:105–113. doi: 10.1016/j.placenta.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Koenig RJ, Lazar MA, Hodin RA, Brent GA, Larsen PR, Chin WW, Moore DD. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989;337:659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- Krassas GE, Poppe K, Glinoer D. Thyroid Function and Human Reproductive Health. Endocr Rev. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- LaFranchi SH, Haddow JE, Hollowell JG. Is thyroid inadequacy during gestation a risk factor for adverse pregnancy and developmental outcomes? Thyroid. 2005;15:60–71. doi: 10.1089/thy.2005.15.60. [DOI] [PubMed] [Google Scholar]
- Laoag-Fernandez JB, Matsuo H, Murakoshi H, Hamada AL, Tsang BK, Maruo T. 3,5,3′-triiodothyronine down-regulates Fas and Fas ligand expression and suppresses caspase-3 and poly (adenosine 5′-diphosphate-ribose) polymerase cleavage and apoptosis in early placental extravillous trophoblasts in vitro. J Clin Endocrinol Metab. 2004;89:4069–4077. doi: 10.1210/jc.2003-032208. [DOI] [PubMed] [Google Scholar]
- Lash GE, Cartwright JE, Whitley GS, Trew AJ, Baker PN. The effects of angiogenic growth factors on extravillous trophoblast invasion and motility. Placenta. 1999;20:661–667. doi: 10.1053/plac.1999.0427. [DOI] [PubMed] [Google Scholar]
- Lash GE, Naruse K, Innes BA, Robson SC, Searle RF, Bulmer JN. Secretion of angiogenic growth factors by villous cytotrophoblast and extravillous trophoblast in early human pregnancy. Placenta. 2010;31:545–548. doi: 10.1016/j.placenta.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F, Huang ST, Funai EF, Schatz F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-alpha and interleukin-1beta in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168:445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubiere LS, Vasilopoulou E, Bulmer JN, Taylor PM, Stieger B, Verrey F, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta. 2010;31:295–304. doi: 10.1016/j.placenta.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Mascanfroni I, Montesinos MM, Susperreguy S, Cervi L, Ilarregui JM, Ramseyer VD, Masini-Repiso AM, Targovnik HM, Rabinovich GA, Pellizas CG. Control of dendritic cell maturation and function by triiodothyronine. FASEB J. 2008;22:1032–1042. doi: 10.1096/fj.07-8652com. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Maruo T, Murata K, Mochizuki M. Human early placental trophoblasts produce an epidermal growth factor- like substance in synergy with thyroid hormone. Acta Endocrinol (Copenh) 1993;128:225–229. doi: 10.1530/acta.0.1280225. [DOI] [PubMed] [Google Scholar]
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- Moreau P, Adrian-Cabestre F, Menier C, Guiard V, Gourand L, Dausset J, Carosella ED, Paul P. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int Immunol. 1999;11:803–811. doi: 10.1093/intimm/11.5.803. [DOI] [PubMed] [Google Scholar]
- Nagamatsu T, Schust DJ. The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci. 2010;17:209–218. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]
- Naruse K, Innes BA, Bulmer JN, Robson SC, Searle RF, Lash GE. Secretion of cytokines by villous cytotrophoblast and extravillous trophoblast in the first trimester of human pregnancy. J Reprod Immunol. 2010;86:148–150. doi: 10.1016/j.jri.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab. 2010;95:E44–E48. doi: 10.1210/jc.2010-0340. [DOI] [PubMed] [Google Scholar]
- Oki N, Matsuo H, Nakago S, Murakoshi H, Laoag-Fernandez JB, Maruo T. Effects of 3,5,3′-triiodothyronine on the invasive potential and the expression of integrins and matrix metalloproteinases in cultured early placental extravillous trophoblasts. J Clin Endocrinol Metab. 2004;89:5213–5221. doi: 10.1210/jc.2004-0352. [DOI] [PubMed] [Google Scholar]
- Otun HA, Lash GE, Innes BA, Bulmer JN, Naruse K, Hannon T, Searle RF, Robson SC. Effect of tumour necrosis factor-alpha in combination with interferon-gamma on first trimester extravillous trophoblast invasion. J Reprod Immunol. 2011;88:1–11. doi: 10.1016/j.jri.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Pang ZJ, Zhou JG, Huang LP. Interleukin-10 may participate in regulating trophoblast invasion in human placentae throughout gestation. Am J Reprod Immunol. 2008;60:19–25. doi: 10.1111/j.1600-0897.2008.00586.x. [DOI] [PubMed] [Google Scholar]
- Prutsch N, Fock V, Haslinger P, Haider S, Fiala C, Pollheimer J, Knofler M. The role of interleukin-1beta in human trophoblast motility. Placenta. 2012;33:696–703. doi: 10.1016/j.placenta.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekhar G, Loganath A, Roy AC, Wong YC. Expression and localization of angiogenin in placenta: enhanced levels at term over first trimester villi. Mol Reprod Dev. 2002;62:159–166. doi: 10.1002/mrd.10116. [DOI] [PubMed] [Google Scholar]
- Renaud SJ, Sullivan R, Graham CH. Tumour necrosis factor alpha stimulates the production of monocyte chemoattractants by extravillous trophoblast cells via differential activation of MAPK pathways. Placenta. 2009;30:313–319. doi: 10.1016/j.placenta.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, Baker PN, Robson SC, Bulmer JN. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012;26:4876–4885. doi: 10.1096/fj.12-210310. [DOI] [PubMed] [Google Scholar]
- Segerer SE, Martignoni F, Bogdan A, Muller N, Kapp M, Dietl J, Rieger L, Kammerer U. Thrombopoietin modulates the proliferation, migration and cytokine profile of decidual cell subsets during early gestation. Mol Hum Reprod. 2013;19:361–368. doi: 10.1093/molehr/gat005. [DOI] [PubMed] [Google Scholar]
- Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilopoulou E, Loubiere LS, Martin-Santos A, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Differential Triiodothyronine Responsiveness and Transport by Human Cytotrophoblasts from Normal and Growth-Restricted Pregnancies. J Clin Endocrinol Metab. 2010;95:4762–4770. doi: 10.1210/jc.2010-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilopoulou E, Loubiere LS, Heuer H, Trajkovic-Arsic M, Darras VM, Visser TJ, Lash GE, Whitley GS, McCabe CJ, Franklyn JA, et al. Monocarboxylate transporter 8 modulates the viability and invasive capacity of human placental cells and fetoplacental growth in mice. PLoS One. 2013;8:e65402. doi: 10.1371/journal.pone.0065402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZM, Yang H, Li M, Yeh CC, Schatz F, Lockwood CJ, Di W, Huang SJ. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta. 2012;33:188–194. doi: 10.1016/j.placenta.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff C, Weigand M, Kreienberg R, Fraser HM. Angiogenesis during primate placentation in health and disease. Reproduction. 2003;126:569–577. doi: 10.1530/rep.0.1260569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.