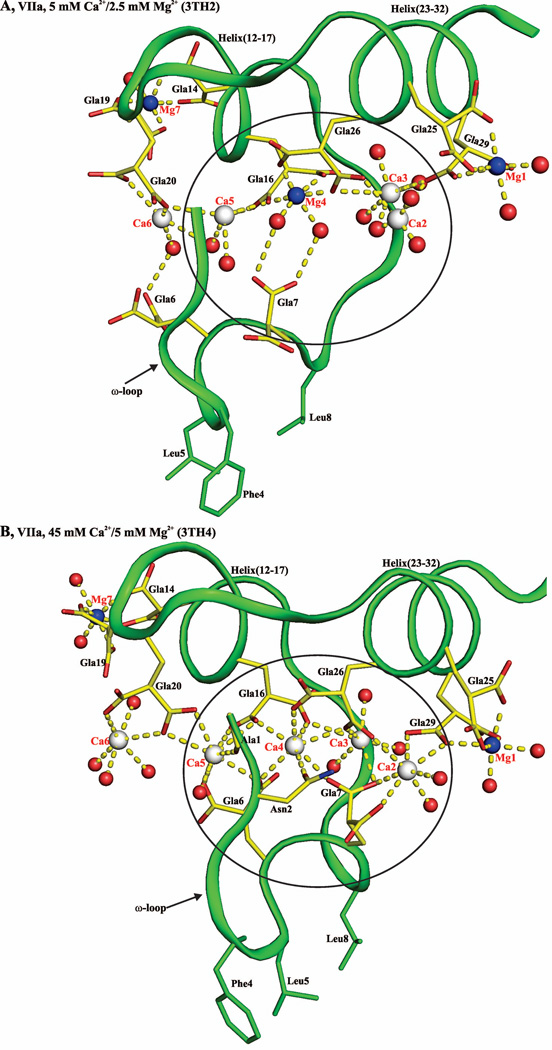
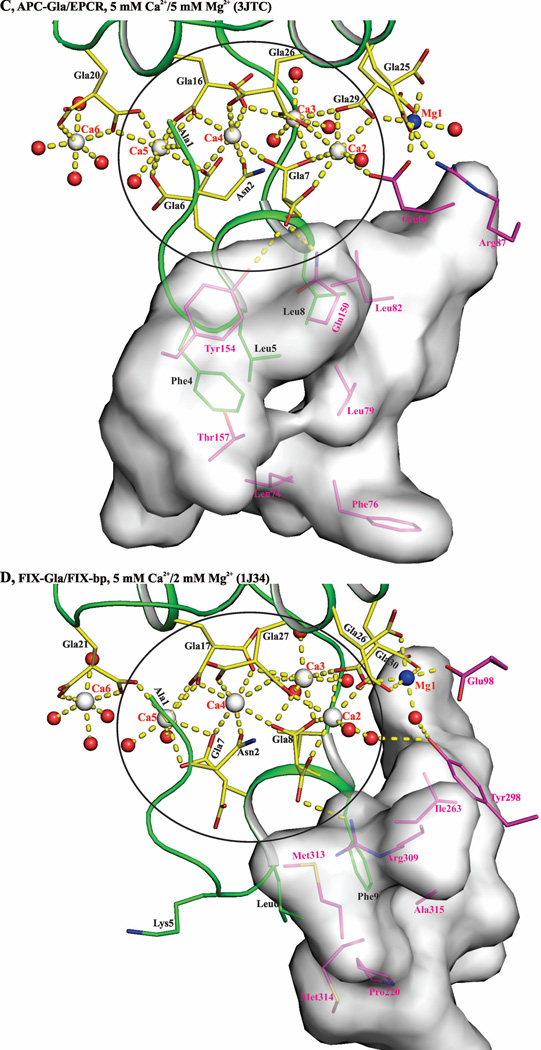
Figure 10. Contributing factors for occupancy of metal position 4 by Mg2+ or Ca2+ in the Gla domains of VKD proteins.
A) Metal ion coordination in the FVIIa-Gla domain under low Mg/low Ca condition. Ca2+ and Mg2+ ions are bound in a linear fashion at the interface between the ω-loop and the two anti-parallel helices. The metal positions 1, 4 and 7 are occupied by Mg2+ and the other four sites are occupied by Ca2+. The metal ions Ca2, Ca3, Mg4, and C5 coordinated to the Gla residues and to the ten water molecules are circled. B) Metal ion coordination in the FVIIa-Gla domain under the low Mg/high Ca. As in ′panel A′, the Ca2+ and Mg2+ ions are bound at the interface between the helical segments and the ω-loop. The metal positions 1, and 7 are occupied by Mg2+ and the other five sites are occupied by Ca2+. The metal ions Ca2, Ca3, Ca4, and Ca5 coordinated to the Gla residues and the six water molecules are circled. C) Metal ion coordination in the APC-Gla domain under low Mg/low Ca condition and its interaction with sEPCR. The sEPCR residues involved in hydrophobic interactions with APC-Gla domain are shown in stick representation. In sEPCR, only the region within 6 Å from the APC-Gla domain hydrophobic residues is shown in surface representation. The metal positions 1 and 7 are occupied by Mg2+ and the other five sites are occupied by Ca2+ as in ′panel B′ for the FVIIa-Gla domain. For clarity, Mg2+ site 7 is not shown. D) Metal ion coordination in the FIX-Gla domain under low Mg/low Ca condition and its interaction with FIX-bp. The FIX-bp residues involved in hydrophobic interactions with FIX-Gla domain are shown in stick representation. In FIX-bp, only the region within 6 Å from the FIX-Gla domain hydrophobic residues is shown in surface representation. The metal positions 1, 7 and 8 (unique to FIX) are occupied by Mg2+ and the other five sites are occupied by Ca2+. Again for clarity, Mg2+ sites 7 and 8 are not shown. The sEPCR and FIX-bp residues are in magenta, whereas the Gla residues are in yellow. The hydrophobic residues present in the ω-loop are in green. The Ca2+, Mg2+ and water molecule are depicted as white, blue and red spheres, respectively. The coordination contacts and the hydrogen bonds are shown in yellow dashed lines.