Table 1. Substitution Effects on the Human and Mouse BRS-3 Activity.
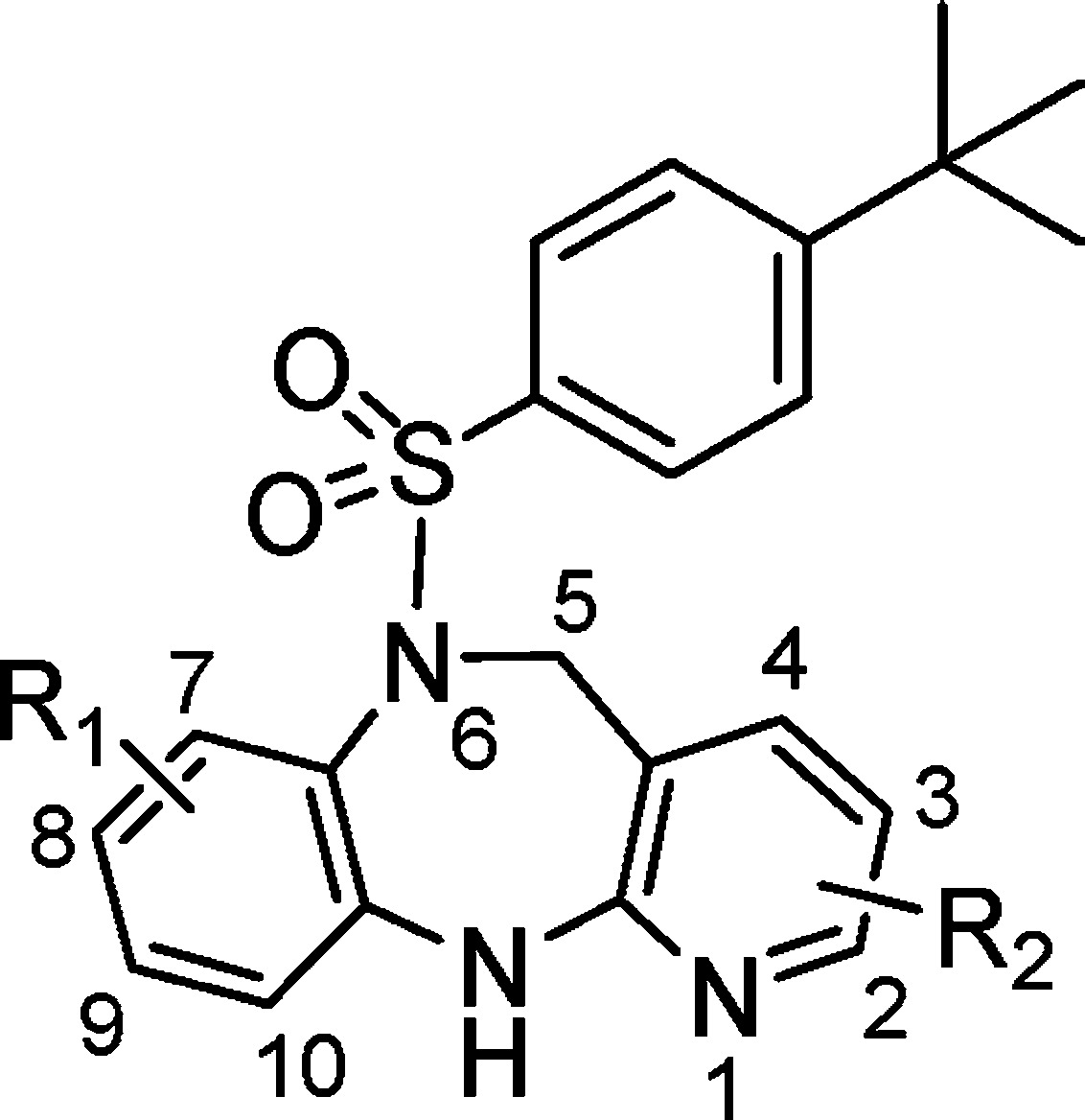
| compda | substitution | hBRS-3b IC50 (nM) | hBRS-3b EC50 (nM) (%Act)c | mBRS-3b EC50 (nM) (% Act)c |
|---|---|---|---|---|
| 1 | 7-Me, 8-Me | 40 ± 21 | 325 ± 117 (91 ± 9%) | 248 ± 42 (101 ± 3%) |
| 7a | 9-Me, 10-Me | 80% inhd at 10 μM | >10000d | >10000d |
| 8a (S) | 7-Me, 8-Me, 2-CF3 | 1.4 ± 0.9 | 97 ± 23 (96 ± 10%) | 33 ± 11 (98 ± 7%) |
| 8b (R) | 7-Me, 8-Me, 2-CF3 | 169 ± 139 | >10000 | >10000 |
| 8c | 7-Me, 8-Me, 2-Me | 10 ± 4.5 | 131 ± 36 (92 ± 13%) | 232 ± 50 (107 ± 10%) |
| 8d (S) | 7-Me, 8-Me, 2-CN | 4.4 ± 1.0 | 50 ± 8 (100 ± 4%) | 50 ± 3 (103 ± 1%) |
| 8e (S) | 7-Me, 8-Me, 2-Cl | 1.2 ± 0.3 | 38 ± 9 (97 ± 2%) | 98 ± 25 (105 ± 7%) |
| 8f | 7-Me, 8-Me, 3-Cl | 430 ± 170 | >10000d | >10000d |
| 8g | 7-Me, 8-Me, 2-Cl, 3-F | 8.9 ± 3.0 | 277 ± 80 (83 ± 1%) | 371 ± 13 (82 ± 6%) |
| 8h (S) | 7-Me, 2-CF3 | 0.9 ± 0.1 | 50 ± 14 (101 ± 10%) | 120 ± 14 (106 ± 1%) |
| 8i (S) | 7-Cl, 2-CF3 | 3.2 ± 0.5 | 63 ± 33 (96 ± 6%) | 155 ± 53 (100 ± 13%) |
| 8j | 8-Me, 2-CF3 | 5.8 ± 0.1 | 199 ± 34 (83 ± 11%) | 377 ± 7 (82 ± 4%) |
| 8k | 2-CF3 | 5.5 ± 0.5 | 110 ± 23 (96 ± 10%) | 161 ± 11 (98 ± 7%) |
| 8l | 7-F, 2-CF3, 8-C(CH3)2OH | 5.0 ± 0.3 | 77 ± 10 (96 ± 2%) | 134 ± 14 (97 ± 0%) |
| 8m (S) | 7-OH, 2-CF3 | 2.2 ± 0.4 | 88 ± 21 (103 ± 1%) | 209 ± 62 (115 ± 14%) |
| 9 | see Figure 4 | 5.3 ± 0.2 | 110 ± 27 (104 ± 0%) | 171 ± 5 (112 ± 12%) |
| 10 (S) | see Figure 4 | 0.7 ± 0.1 | 108 ± 44 (101 ± 8%) | 129 ± 18 (107 ± 4%) |
Racemic mixture or achiral compound (7, 8j, 8k, etc.), except as noted.
Data expressed as mean ± SD (n ≥ 2 independent experiments).
% Act represents the maximum activation of tested compound relative to that of the dY peptide ([d-Tyr,6β-Ala,11 Phe,13 and Nle14]-bombesin(6–14)).
Assayed only once.
