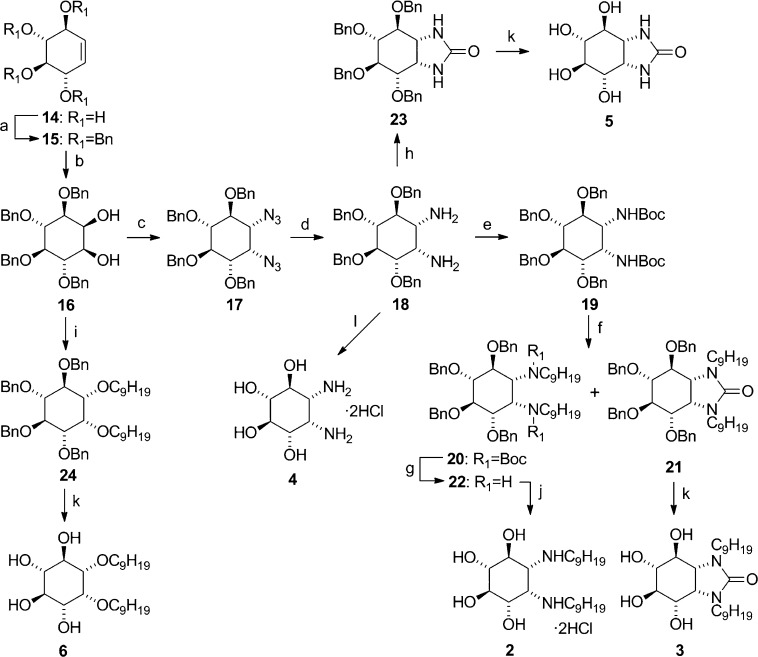
Scheme 2. Synthesis of Compounds 2–6 from (+)-Conduritol B 14.
Reagents and conditions: (a) NaH, BnBr, DMF, 30 °C, 75%. (b) OsO4, acetone–H2O, N-methylmorpholine N-oxide, rt, 83%. (c) (1) MsCl, pyridine, rt; (2) NaN3, DMF, 85 °C, 77% for two steps. (d) LiAlH4, THF, rt, 93%. (e) Boc2O, Et3N, CH2Cl2, rt, 53%. (f) NaH, C9H19I, DMF, 80 °C, 21% (20), 24% (21). (g) CF3CO2H, CH2Cl2, rt, 86%. (h) N,N′-carbonyldiimidazole, CH2Cl2, reflux, 83%. (i) NaH, C9H19I, DMF, 0 °C, 78%. (j) Pd/C, MeOH, HCl, H2 (2 atm), rt, 85%. (k) Pd/C, MeOH, H2 (2 atm), rt, 88–93%. (l) BCl3, CH2Cl2, −78 °C, 87%.