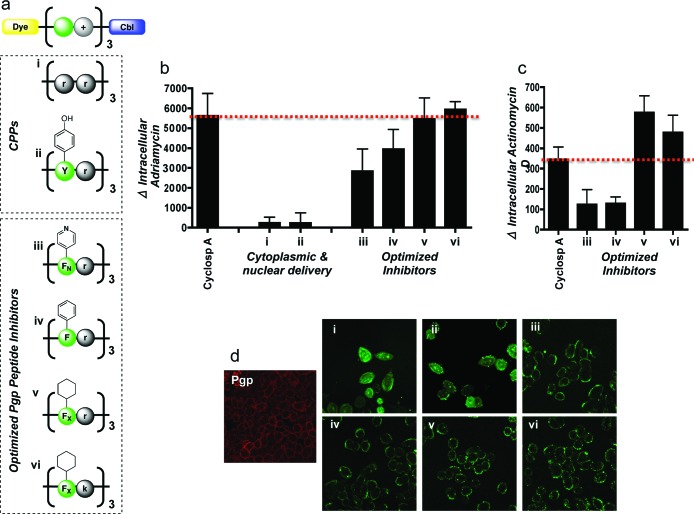
Figure 2.
Evaluation of peptide-Cbl physicochemical properties on Pgp activity. (a) Structures of the panel of fluorescently labeled peptide-Cbl conjugates used for optimization of the Pgp inhibitor. d-Enantiomers were used for all arginines (r) and lysines (k) to reduce proteolytic cleavage. Pyridinylalanine and cyclohexylalanine are denoted as FN and FX, respectively. (b) Change in intracellular levels of adriamycin following pretreatment with a 5 μM concentration of various peptide-Cbl conjugates. Analysis was done by flow cytometry in live A2780 cells overexpressing Pgp efflux pumps. Mean values are plotted, n = 3; error bars are SEM. Peptide-Cbl conjugates show Pgp inhibition at comparative levels to cyclosporine A. See Figure S2 in the Supporting Information for basal levels of uptake. (c) Change in intracellular levels of actinomycin D following pretreatment with a 5 μM concentration of various peptide-Cbl conjugates. Mean values are plotted, n = 3; error bars are SEM. See Figure S3 in the Supporting Information for basal levels of uptake. (d) Intracellular localization of fluorescently labeled peptide-Cbl conjugates (5 μM) visualized by confocal microscopy in live cells. Immunofluorescence was used to visualize basal Pgp expression in AdrR cells (left image, red). Intracellular localization of conjugates iii−vi closely matched the staining pattern of the basal Pgp, suggesting that these conjugates were localizing to the efflux pumps. These results illustrate that inhibition efficacy is dependent on plasma membrane localization.