Table 1. Structures and KHK Inhibition Results for Derivatives of 1 with Variation of R1.
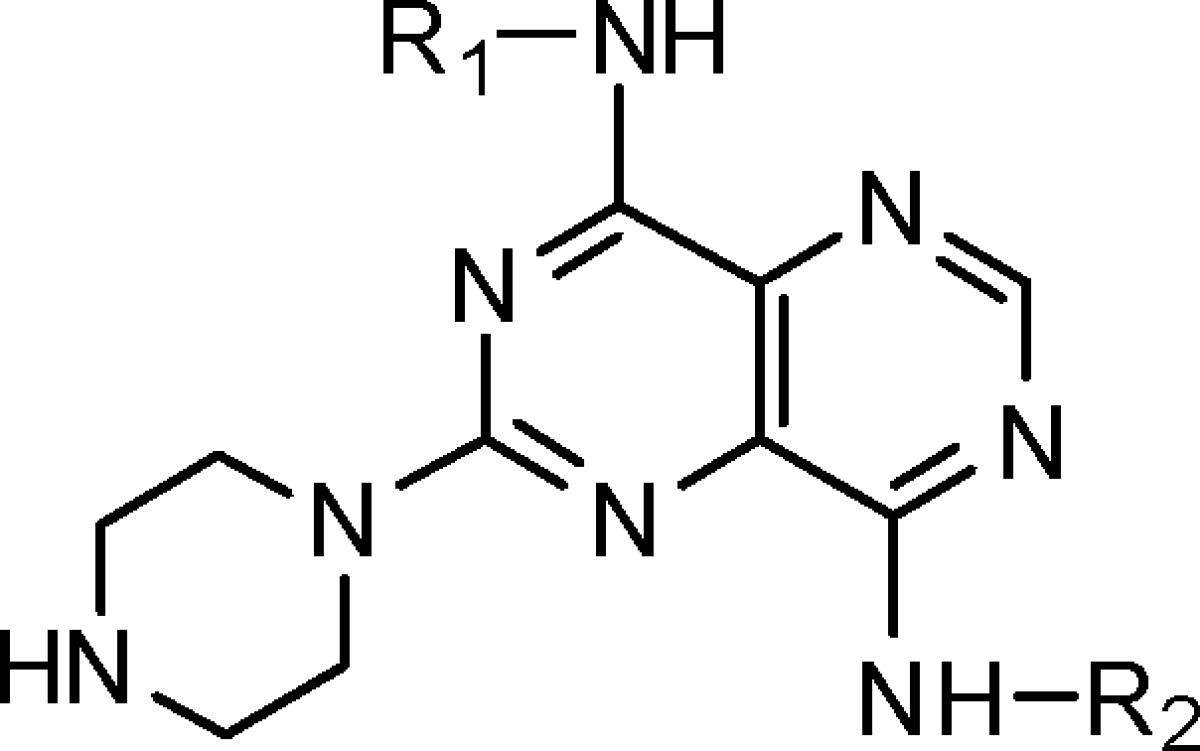
| compda | R1 | R2 | IC50 (nM)b |
|---|---|---|---|
| 2 | 2-MeC6H4 | Me | 400 |
| 3 | 2-MeC6H4 | CH2-c-Pr | 210 |
| 4 | Ph | CH2-c-Pr | 3200 |
| 5 | 3-MeC6H4 | CH2-c-Pr | 2800 |
| 6 | 2-MeOC6H4 | CH2-c-Pr | 100 |
| 7 | 2-EtOC6H4 | CH2-c-Pr | 200 |
| 8 | 2-MeSC6H4 | CH2-c-Pr | 12 |
| 9 | 3-MeSC6H4 | CH2-c-Pr | 2000 |
| 10 | 4-MeSC6H4 | CH2-c-Pr | >9000 |
| 11 | 2-MeSO2C6H4 | CH2-c-Pr | >9000 |
| 12 | 2-EtSC6H4 | CH2-c-Pr | >9000 |
| 13 | 2-CF3SC6H4 | CH2-c-Pr | 3000 |
| 14 | 2-EtC6H4 | CH2-c-Pr | 130 |
| 15 | 2-(i-Pr)C6H4 | CH2-c-Pr | 5000 |
| 16 | 2-(c-Pr)C6H4 | CH2-c-Pr | 380 |
| 17 | 2-FC6H4 | CH2-c-Pr | 1500 |
| 18 | 2-ClC6H4 | CH2-c-Pr | 540 |
| 19 | 2-BrC6H4 | CH2-c-Pr | 170 |
| 20 | c-Pr | CH2-c-Pr | >9000 |
| 21 | c-hexyl | CH2-c-Pr | >9000 |
New compounds were purified by HPLC and characterized by ESI-MS and 1H NMR (see the Supporting Information).
Inhibition of recombinant human hepatic KHK (KHK-C) in terms of IC50 values; >9000 nM relates to <50% inhibition at 9 μM.
