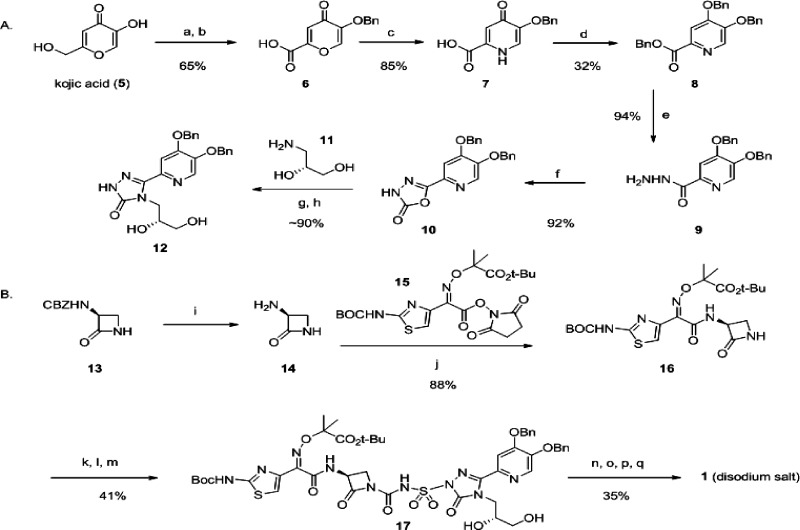
Scheme 1. Representative Monocarbam Synthesis: Preparation of MC-1 (1),
(A) Preparation of the substituted triazalone intermediate 12. Reagents and conditions: (a) Benzylchloride, K2CO3, DMF. (b) CrO3, H2SO4, H2O/acetone, −5 °C to room temperature. (c) NH4OH, H2O, 80 °C. (d) Benzylchloride, K2CO3, DMF, 80 °C. (e) Hydrazine monohydrate, methanol, 65 °C. (f) Carbonyldiimidazole, THF. (g) Compound 11, THF, 60 °C. (h) Aqueous KOH, 100 °C.
(B) Construction of the monocarbam moiety followed by stepwise deprotection leading to 1 disodium salt. Reagents and conditions: (i) H2, 10% Pd/C, toluene/ethanol (assumed quant., used directly in 20% theoretical excess). (j) Toluene/ethanol, 5 h. (k) MSTFA, THF, 12, concentrate at 60 °C. (l) ClSO2NCO, 16, CH2Cl2, 0 °C. (m) Add 12 in fresh THF to 16, 0 °C to room temperature. (n) Pd black, H2, acetic acid, THF. (o) Trifluoroacetic acid, CH2Cl2, 0 °C. (p) NaHCO3 (2 equiv), H2O. (q) Freeze at −80 °C and lyophilize.