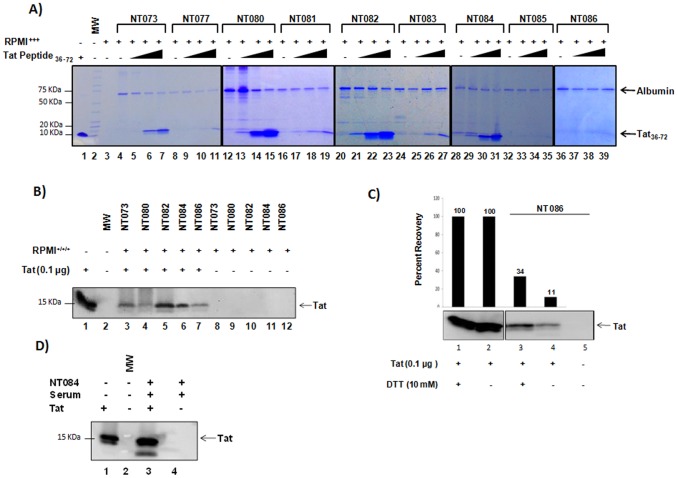
Figure 1. Capture of Tat protein by nanotrap particles.
(A) Tat36–72 peptide was spiked into RPMI-1640 containing FBS, antibiotics and glutamine (RPMI+++) at 0.1 µg (lanes 5, 9, 13, 17, 21, 25, 29, 33, 37), 1 µg (lanes 6, 10, 14, 18, 22, 26, 30, 34, 38), and 10 µg (lanes 7, 11, 15, 19, 23, 27, 31, 35, 39). A 30% slurry of nanotrap particles prepared in unsupplemented RPMI was incubated with RPMI containing Tat36–72 for 30 min at room temperature. Eluted materials (15 µl) were then coomassie stained for the presence of captured Tat peptide. (B) Full-length Tat protein spiked in RPMI-1640 (+++) (100 µl) was captured by a 30% slurry of each nanotrap particle in unsupplemented RPMI (lanes 3–7), separated on SDS-PAGE and detected by Western blot using anti-tat antibody. Nanotrap particles incubated with RPMI+++ without Tat were used as background control (lanes 8–12). (C) Full-length Tat was pre-treated with DTT at 37°C for 30 min (lanes 1 and 3) and incubated with NT086 (lane 3). Percent recovery Tat capture under reducing conditions was determined from raw densitometry counts of the western blot. (D) Tat protein (1 µg) was spiked into uninfected patient serum (100 µl), incubated with NT084 (lane 3), and subsequent Tat capture determined by western blot.