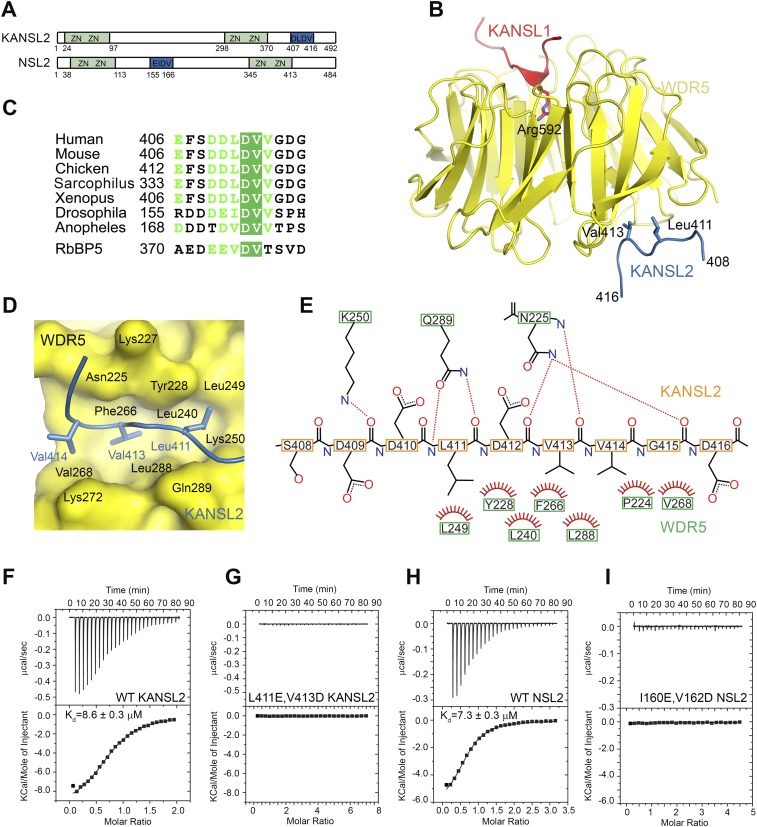
Figure 2.
Structural basis for the WDR5–KANSL2 interaction. (A) Schematic representation of the domain structure of human and Drosophila KANSL2/NSL2. KANSL2 contains four putative Zn-coordinating motifs and an additional WDR5-binding motif (shown in blue) identified in this study. (B) Ribbon diagram of the minimal human KANSL1–WDR5–KANSL2 complex structure. WDR523–334 is shown in yellow, KANSL1 is in red, and KANSL2 is in blue. The key interacting residues are shown as sticks. (C) Sequence alignment of NSL2 proteins. Only the sequence WDR5-binding fragment is shown. The corresponding sequence of the RbBP5- and WDR5-binding motif is aligned with KANSL2. (D) Details of the WDR5–KANSL2 interaction interface. KANSL2 Leu411, Val413, and Val414 insert into a wide hydrophobic pocket on WDR5. (E) Schematic representation of the interactions between KANSL2 and WDR5. Hydrogen bonds are denoted with dotted lines. Hydrogens are not shown for clarity. (F,G) ITC measurement of the interaction between WDR523–334 and KANSL2 WDR5-binding peptide (Y-406-EFSDDLDVVGDG-417) (F) and its mutated version (Y-406-EFSDDEDDVGDG-417) (G). (H,I) ITC measurement of the interaction between WDS50–361 and the NSL2 WDS-binding peptide (Y-155-RDDDEIDVVSPH-166) (H) and its I160E/V162D mutant (Y-155-RDDDEEDDVSPH-166) (I).