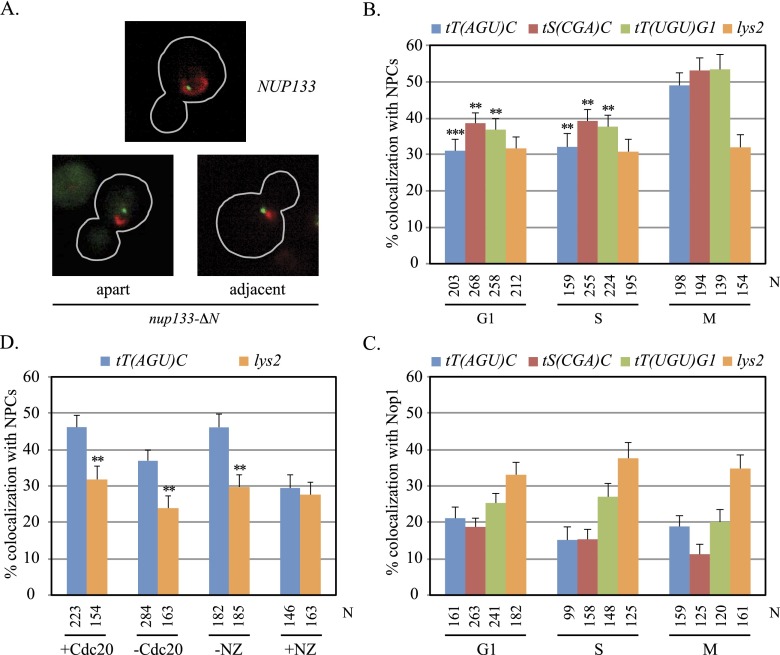
Figure 1.
Localization of tDNAs at NPCs. (A) Representative images of cells from asynchronously grown cultures. GFP-lac repressor (green) and Nic96-mRFP (red) are shown in nup133-ΔN and NUP133 cells, outlined in gray. (B) tDNA localization at NPCs. Asynchronously grown cultures of strains MC78 [tT(AGU)C∷256lacop], MC197 [tS(CGA)C∷256lacop], MC198 [tT(UGU)G1∷256lacop], and MC180 (lys2∷256lacop) were used. N equals the number of cells examined. Localization of each tDNA at NPCs was significantly higher in M phase than in G1 or S. Pairwise χ2-tests, (***) <5 × 10−4; (**) <5 × 10−3. (C) tDNAs colocalized with the nucleolus rarely. Strains MC250 [tT(AGU)C∷256lacop], MC247 [tS(CGA)C∷256lacop], MC248 [tT(UGU)G1∷256lacop], and MC249 (lys2∷256lacop) bearing a Nop1-CFP expression plasmid (pJW1327) were examined in asynchronous cultures. The specific tDNAs examined here differ from those described in Thompson et al. (2003). (D) NPC-tT(AGU)C colocalization in M phase. Strains MC78 [tT(AGU)C∷256lacop] and MC180 (lys2∷256lacop) bearing MET3p-CDC20 were examined before and after depletion of Cdc20. Strains MC64 [tT(AGU)C∷256lacop] and MC226 (lys2∷256lacop) were examined before and after addition of nocodazole (NZ). In the absence of either arrest, only M-phase cells were scored. NPC localization was significantly higher for tT(AGU)C than lys2 where noted.