Figure 1.
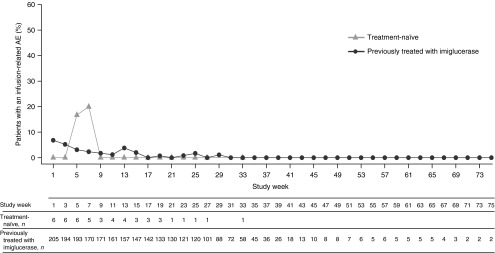
Percentage of safety-assessment patient population experiencing infusion-related adverse events (AEs) by study week. An infusion-related AE was defined as an AE that began either during or within 12 h after the start of the infusion and was judged as possibly/probably related to study drug.
