Abstract
Peri-procedural hyperglycemia is an independent predictor of mortality in patients undergoing percutaneous coronary intervention (PCI). However, peri-procedural management of blood glucose is not standardized. The effects of routinely continuing long-acting glucose-lowering medications prior to coronary angiography with possible PCI on peri-procedural glycemic control have not been investigated. Patients with diabetes mellitus (DM) (n=172) were randomized to continue (Continue group; n=86) or hold (Hold group; n=86) their clinically prescribed long-acting glucose-lowering medications prior to procedure. The primary endpoint was glucose level on procedural access. In a subset of patients (no DM group, n=25, Continue group, n=25, and Hold group, n=25), selected measures of platelet activity that change acutely were assessed. Patients with DM randomized to the Continue group had lower blood glucose levels on procedural access compared with those randomized to the Hold group (117 [97–151] vs 134 [117–172] mg/dL, p=0.002). There were 2 hypoglycemic events in the Continue group and none in the Hold group, and no adverse events in either group. Selected markers of platelet activity differed across the no DM, Continue, and Hold groups (leukocyte platelet aggregates: 8.1% [7.2–10.4], 8.7% [6.9–11.4], 10.9% [8.6–14.7], p=0.007; monocyte platelet aggregates: 14.0% [10.3–16.3], 20.8% [16.2–27.0], 22.5% [15.2–35.4], p<0.001; soluble p-selectin: 51.9ng/mL [39.7–74.0], 59.1ng/mL [46.8–73.2], 72.2ng/mL [58.4–77.4], p=0.014). In conclusion, routinely continuing clinically prescribed long-acting glucose-lowering medications prior to coronary angiography with possible PCI helps achieve peri-procedural euglycemia, appears safe, and should be considered as a strategy for achieving peri-procedural glycemic control.
Keywords: hyperglycemia, diabetes mellitus, coronary angiography, percutaneous coronary intervention
Peri-procedural hyperglycemia predicts adverse events and mortality in patients undergoing percutaneous coronary intervention (PCI) (1–2). Some data suggest that metabolic interventions during PCI may prevent deleterious effects of hyperglycemia (3–5).However, glycemic control has not been consistently achieved in trials using intravenous insulin, so not all trials have demonstrated a reduction in clinical events (6–8). There is wide variability in the management of long-acting glucose-lowering medications prior to coronary angiography and PCI in patients with diabetes mellitus (DM) (9). Furthermore, in the current era of coronary angiography and PCI, where procedure times are shorter, sedation is minimal, and patients are able to eat shortly after the procedure, it is not certain if there is any need to routinely hold long-acting glucose-lowering medications prior to procedure as done in surgical populations. Accordingly, in this randomized clinical trial, we sought to evaluate whether routinely continuing clinically prescribed long-acting glucose-lowering medications in patients with type 2 DM prior to coronary angiography with possible PCI effectively achieves peri-procedural euglycemia. Since hyperglycemia is associated with increased platelet activity, we also explored the effect of continuing long-acting glucose-lowering medications on relevant measures of platelet activity (10).
Methods
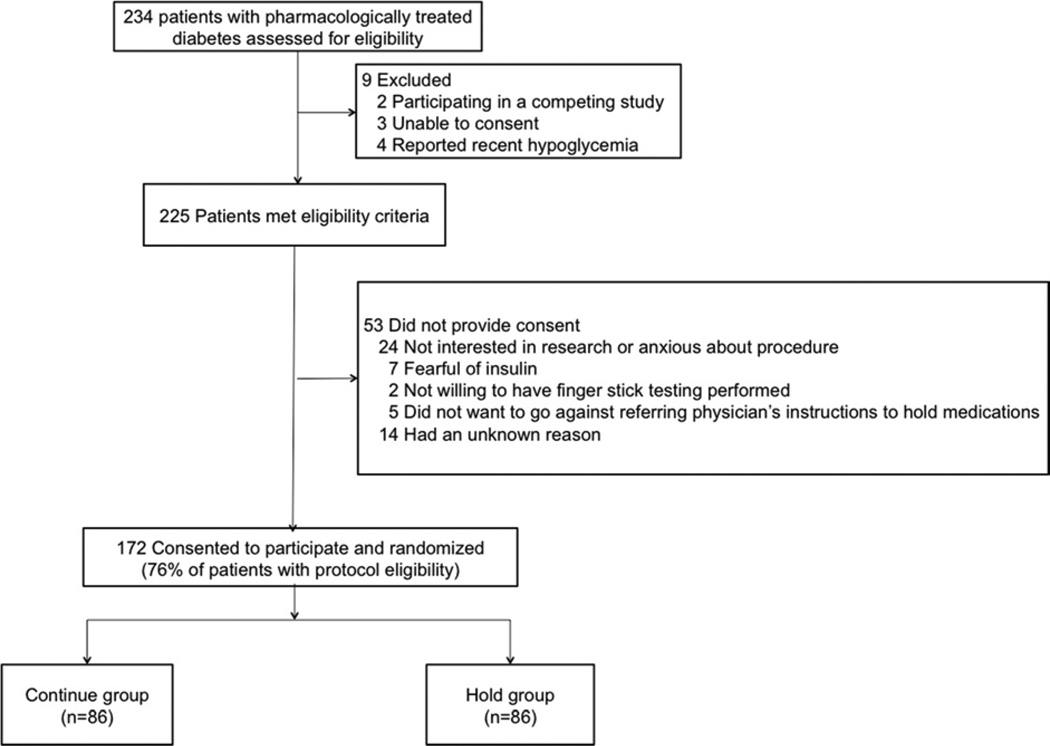
All patients with type 2 DM referred for coronary angiography with possible PCI at the Manhattan Veterans Affairs hospital were eligible to participate in the study. Patients were excluded if they were unable to give consent, were participating in a competing study, or had a history of symptomatic hypoglycemia (Figure 1). All patients provided written, informed consent. This study was approved by the institutional review board and registered at ClinicalTrials.gov (identifier: NCT01419652).
Figure 1.
Screening, enrollment, and randomization of study population
Randomization was performed in a 1:1 manner with sealed opaque envelopes using random block sizes of 4 and 8 (11). Patients randomized to the Continue group were instructed to continue their clinically prescribed long-acting oral and injectable glucose-lowering medications as usual prior to procedure. Patients prescribed metformin were instructed to continue their pre-procedural dose as scheduled but not to resume therapy until 48 hours following the procedure as per package insert. Patients randomized to the Hold group were instructed to hold all oral and injectable glucose-lowering medications the morning of the procedure (including glargine insulin the night prior procedure if prescribed). Blood glucose levels were not monitored routinely pre-procedure, but only if patients expressed not feeling well. Upon procedural access, all patients had arterial blood collected via the sheath prior to any medication administration for assessment of blood glucose by point-of-care glucometer.
The primary endpoint was blood glucose level at the time of procedural access in the Continue versus Hold group. The safety endpoint was the rate of hypoglycemic events (asymptomatic glucose<50mg/dL or symptomatic glucose <75mg/dL) (12–13).
A platelet sub-study (n=75) was performed with 25 comparator patients with no history of DM, 25 patients from the Continue group, 25 patients from the Hold group. Patients in the platelet sub-study were either on aspirin therapy alone or on dual anti-platelet therapy with aspirin and clopidogrel prior to procedural access. Patients were excluded if they took cilostazol, dipyridamole, any non-steroidal anti-inflammatory drugs other than aspirin, or any other anti-platelet agent as part of their prescribed medication regimen. Citrate-anticoagulated blood was collected at procedural access from the arterial sheath (minimum 5 french) after an initial 2cc discard for assessment of the following measures of platelet activity: monocyte (MPA) and leukocyte platelet aggregates (LPA) and soluble p-selectin. These markers of platelet activity were chosen based on their association with in vivo platelet activation, as well as their ability to change acutely and, thereby, may reflect acute changes in platelet activity caused by differences in glucose levels. MPA and LPA were measured via flow cytometery using directly conjugated CD14-PE, CD 45-PE and CD42a-FITC antibodies. Measurements of soluble p-selectin were made using commercial enzyme-linked immunosorbent assay (eBioscience).
Sample size was based on observational data in the catheterization laboratory at the Manhattan Veterans hospital (mean glucose at procedural access in patients with DM of 154 ± standard deviation 61 mg/dL) and an estimated decrease of mean glucose by 20% when continuing long-acting glucose-lowering medications. Glucose values on a log scale were normally distributed and used to calculate sample size (4.97 ± 0.36 mg/dL; estimated 3.2% decrease). Using a two-sided two-sample t-test, approximately 82 patients with DM were needed in each group (Continue and Hold groups) to achieve 80% power at the 0.05 significance level (PASS 2008. NCSS, LLC. Kaysville, Utah).
Categorical variables are presented as proportions and compared using tests of proportions, while skewed continuous variables (Shapiro-Wilks test) are presented as median [interquartile range] and compared using Mann Whitney test and Kruskal-Wallis test for 2- and 3-way comparisons. Confidence intervals for number of hyperglycemic events in each group were presented using the Wilson score interval. A significance of 0.05 was set for the primary comparison. Due to the exploratory nature of platelet activity testing and multiple measurements, a significance of 0.01 was set for comparisons of markers of platelet activity.
Results
Baseline characteristics of patients randomized to the Continue (n=86) and Hold groups (n=86) are presented in Table 1. There were no major differences in baseline characteristics.
Table 1.
Baseline characteristics of total cohort
| Variable | Patients with Diabetes Mellitus | p-value | |
|---|---|---|---|
| Continue group (n=86) | Hold group (n=86) | ||
| Age (years) | 64 [60–74] | 66 [62–73] | 0.25 |
| Men | 85 (99%) | 86 (100%) | 1.0 |
| White | 62 (72%) | 63 (73%) | 0.58 |
| Black | 17 (20%) | 13 (15%) | |
| Hispanic | 7 (8%) | 10 (12%) | |
| Body mass index (kg/m2) | 32 [29–36] | 30 [27–35] | 0.08 |
| Abdominal circumference (inch) | 46 [42–50] | 44 [40–48] | 0.03 |
| Previous myocardial infarction | 23 (27%) | 26 (30%) | 0.74 |
| Hypertension* | 81 (94%) | 81 (94%) | 1.0 |
| Hyperlipidemia* | 77 (90%) | 78 (91%) | 1.0 |
| Chronic kidney disease | 19 (22%) | 13 (15%) | 0.33 |
| Previous stroke | 15 (17%) | 13 (15%) | 0.84 |
| Peripheral vascular disease | 10 (12%) | 17 (20%) | 0.21 |
| Tobacco use | 27 (32%) | 17 (20%) | 0.11 |
| Presenting with acute coronary syndrome | 18 (21%) | 25 (29%) | 0.29 |
| Sulfonylurea | 37 (43%) | 39 (45%) | 0.88 |
| Metformin | 52 (61%) | 53 (62%) | 1.0 |
| Thiazoladinediones | 6 (7%) | 4 (5%) | 0.75 |
| Sitagliptin | 3 (4%) | 0 | 0.25 |
| Long-acting insulin | 39 (45%) | 35 (41%) | 0.64 |
| Multiple glucose-lowering agents | 40 (47%) | 35 (41%) | 0.54 |
| Oral agents only With insulin |
15 (38%) 25 (63%) |
17 (49%) 18 (51%) |
0.36 |
| Ejection fraction | 0.64 | ||
| Normal | 64 (76%) | 63 (74%) | |
| Severely reduced | 6 (7%) | 4 (5%) | |
| Random glucose (mg/dL) | 141 [108–197] | 146 [120–186] | 0.55 |
| Hemoglobin A1c (%) | 7.1 [6.5–8.5] | 7.3 [6.8–8.0] | 0.94 |
| Glomerular filtration rate (mL/min) | 70 [54–84] | 72 [59–98] | 0.08 |
| Low density lipoprotein-cholesterol (mg/dL) | 74 [59–92] | 74 [61–94] | 0.66 |
| High density lipoprotein-cholesterol (mg/dL) | 39 [33–45] | 37 [32–45] | 0.96 |
| Triglyceride (mg/dL) | 151 [106–211] | 145 [105–214] | 0.81 |
| Fasting time (hours) | 16.1 [14.0–19.2] | 17.3 [14.4–19.3] | 0.41 |
Hypertension and hyperlipidemia are defined as self-reported history of or documentation of these diagnoses in a physician note in the electronic medical record system.
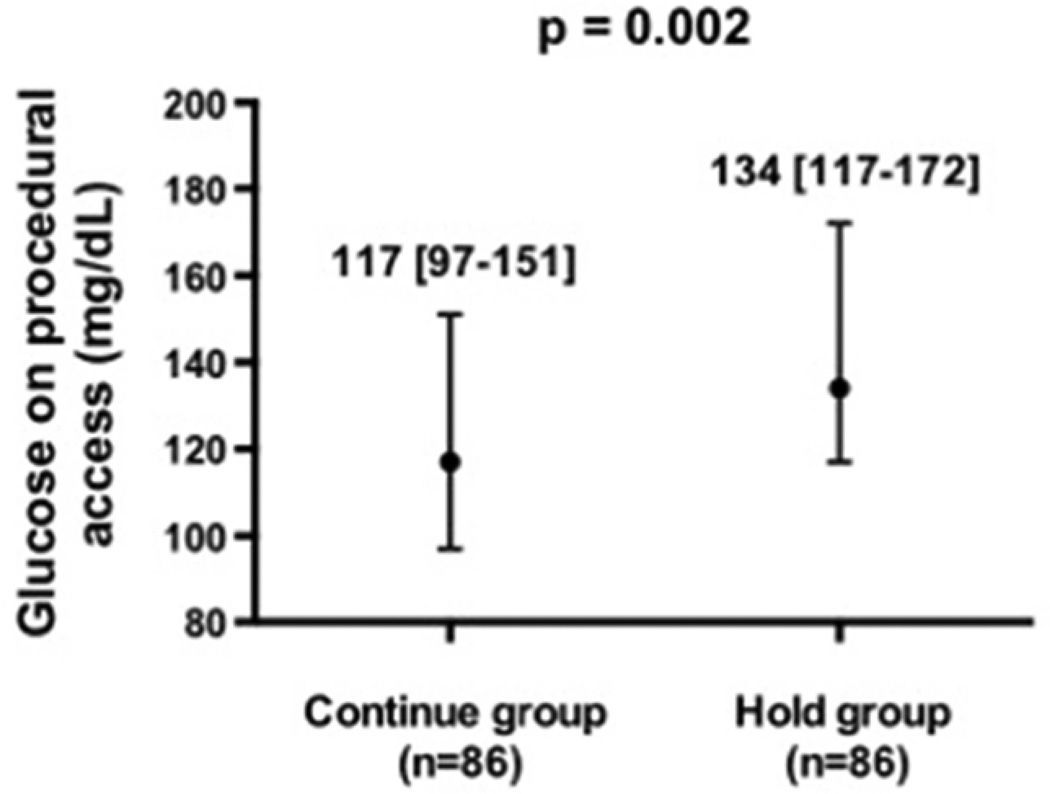
Blood glucose on procedural access was significantly lower in patients randomized to the Continue vs Hold group (Figure 2). There were 2 (0.01,0.08) hypoglycemic events in the Continue group and 0 (0,0.04) in the Hold group (p=0.50). One event was an asymptomatic blood glucose of 43 mg/dL on procedural access, treated with intravenous glucose. This patient was on long-acting insulin, metformin and glyburide prior to angiography. The second event was a blood glucose of 48 mg/dL 3 hours prior to procedure associated with perspiration and treated successfully with orange juice. This patient was on long-acting insulin, metformin and glipizide prior to procedure and had a glucose of 167mg/dL on procedural access.
Figure 2.
Glucose level at time of procedural access in patients with diabetes mellitus randomized to continue versus hold prescribed long-acting glucose-lowering medications.
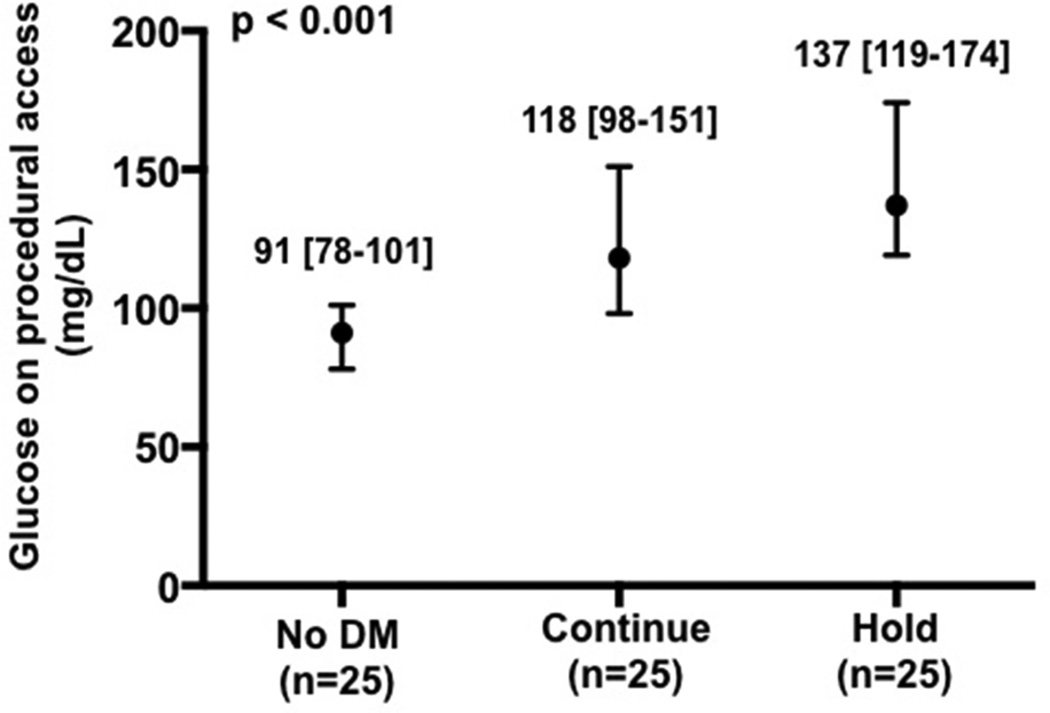
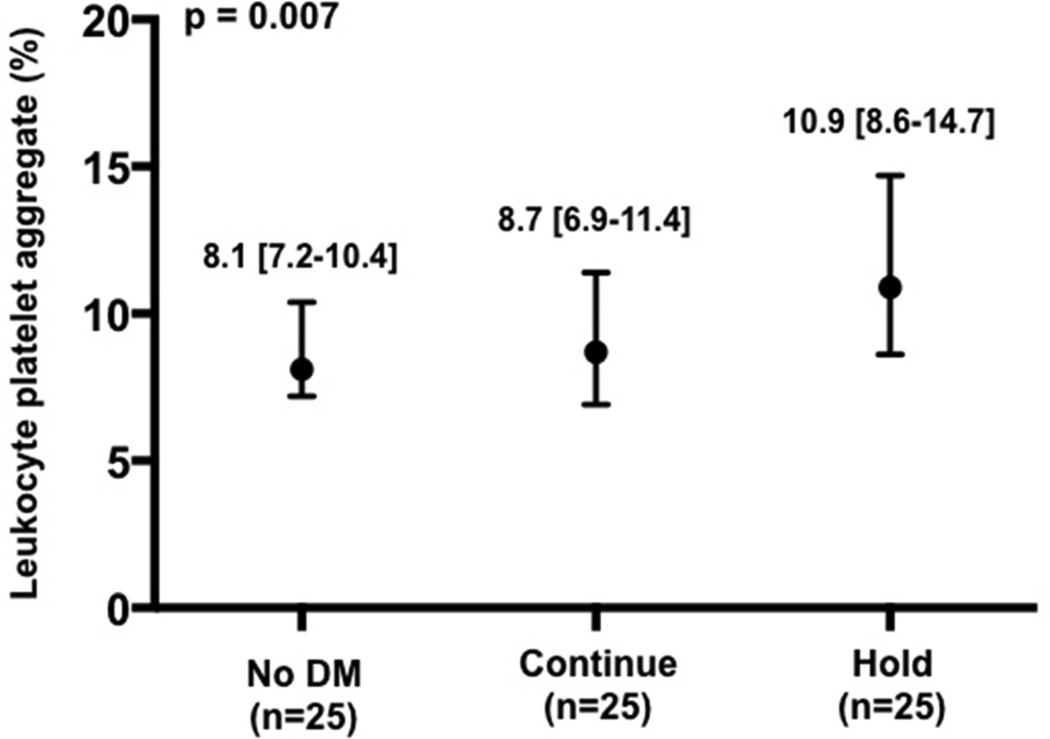
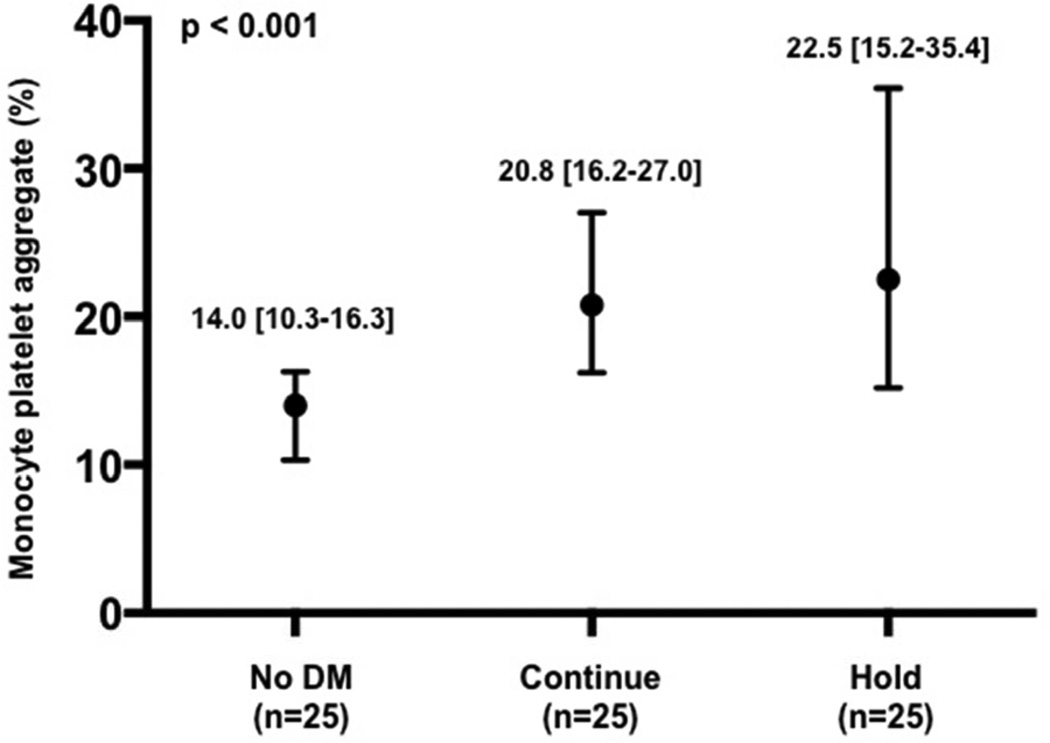
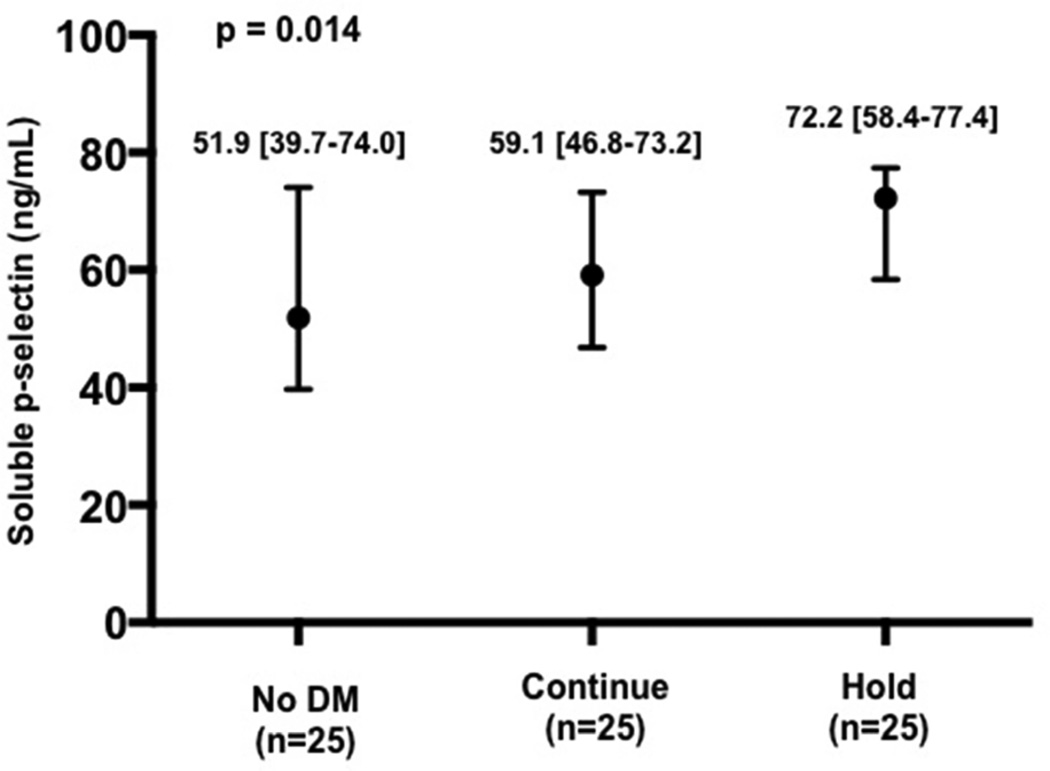
Baseline characteristics of the patients enrolled in the platelet sub-study are presented in Table 2. As expected, median glucose at the time of procedural access was higher in the Hold compared with Continue and without DM groups (Figure 3a). Platelet activity at the time of procedural access, as demonstrated by LPA, MPA, and soluble p-selectin levels, was also higher in the Hold compared with Continue and no DM groups (Figure 3b–d).
Table 2.
Baseline characteristics of patients in the platelet sub-study
| No Diabetes Mellitus (n=25) |
Continue group (n=25) |
Hold group (n=25) |
p- value* |
p- value** |
|
|---|---|---|---|---|---|
| Age (years) | 65 [61–77] | 65 [63–71] | 68 [63–73] | 0.52 | 0.28 |
| Men | 23 (92%) | 25 (100%) | 25 (100%) | 0.13 | 1.0 |
| White | 20 (80%) | 19 (76%) | 20 (80%) | 0.38 | 0.34 |
| Black | 5 (20%) | 4 (16%) | 5 (20%) | ||
| Hispanic | 0 | 2 (8%) | 0 | ||
| Body mass index (kg/m2) | 29 [27–32] | 34 [30–36] | 30 [27–33] | 0.03 | 0.04 |
| Abdominal circumference (inch) | 40 [37–44] | 47 [44–52] | 44 [39–47] | 0.001 | 0.03 |
| Previous myocardial infarction | 5 (20%) | 10 (40%) | 7 (28%) | 0.30 | 0.55 |
| Hypertension | 20 (80%) | 24 (96%) | 22 (88%) | 0.22 | 0.61 |
| Hyperlipidemia | 18 (72%) | 22 (88%) | 24 (96%) | 0.05 | 0.61 |
| Chronic kidney disease | 2 (8%) | 5 (20%) | 5 (20%) | 0.41 | 1.0 |
| Previous stroke | 3 (12%) | 4 (16%) | 2 (8%) | 0.68 | 0.67 |
| Peripheral vascular disease | 4 (16%) | 2 (8%) | 4 (16%) | 0.63 | 0.67 |
| Tobacco use | 7 (28%) | 5 (20%) | 5 (20%) | 0.74 | 1.0 |
| Presenting with acute coronary syndrome | 5 (20%) | 5 (20%) | 7 (28%) | 0.74 | 0.74 |
| Dual anti-platelet therapy with aspirin and clopidogrel prior to arterial access | 14 (56%) | 13 (52%) | 16 (64%) | 0.80 | 0.65 |
| Sulfonylurea | -- | 11 (44%) | 12 (48%) | -- | 1.0 |
| Metformin | -- | 16 (64%) | 16 (64%) | -- | 1.0 |
| Thiazoladinediones | -- | 2 (8%) | 0 | -- | 0.49 |
| Sitagliptin | -- | 2 (8%) | 0 | -- | 0.49 |
| Long-acting nsulin | -- | 9 (36%) | 10 (40%) | -- | 1.0 |
| Ejection fraction | 0.33 | 0.18 | |||
| Normal | 18 (72%) | 16 (67%) | 21 (88%) | ||
| Severely reduced | 2 (8%) | 7 (29%) | 1 (4%) | ||
| Random glucose (mg/dL) | 90 [83–96] | 141 [109–168] | 144 [116–182.] | <0.001 | 0.67 |
| Hemoglobin A1c (%) | 5.8 [5.5–6.0] | 7.1 [6.4–7.9] | 7.0 [6.8–7.8] | <0.001 | 0.78 |
| Platelets (× 109/L) | 198 [139–236] | 193 [171–244] | 194 [169–228] | 0.84 | 0.85 |
| Mean platelet volume (fL) | 9.1 [8.5–9.7] | 8.7 [7.7–9.3] | 9.0 [7.9–9.6] | 0.34 | 0.52 |
| Glomerular filtration rate (mL/min) | 90 [69–140] | 80 [54–89] | 72 [57–96] | 0.19 | 0.69 |
| Low density lipoprotein-cholesterol (mg/dL) | 91 [70–140] | 71 [55–86] | 69 [56–82] | 0.008 | 0.98 |
| High density lipoprotein-cholesterol (mg/dL) | 41 [36–45] | 38 [31–46] | 35 [32–51] | 0.28 | 0.58 |
| Triglyceride (mg/dL) | 133 [85–199] | 138 [109–197] | 154 [106–186] | 0.84 | 0.96 |
| Number of coronary arteries narrowed | |||||
| None | 8 (32%) | 5 (20%) | 7 (28%) | 0.33 | 0.33 |
| 1 vessel | 6 (24%) | 8 (32%) | 3 (12%) | ||
| 2 vessel | 3 (12%) | 8 (32%) | 8 (32%) | ||
| 3 vessel | 8 (32%) | 4 (16%) | 7 (28%) | ||
| Fasting time (hours) | 17.8 [15.1–18.9] | 16.0 [13.5–18.5] | 16.8 [15.6–18.2] | 0.45 | 0.36 |
p-value comparing all three groups;
p-value comparing continue versus hold groups
Figure 3.
Glucose (a), leukocyte platelet aggregates (b), monocyte platelet aggregates (c), and soluble p-selectin (d) at time of procedural access in patients with no diabetes mellitus (DM) and patients with DM randomized to continue versus hold prescribed long-acting glucose-lowering medications.
Discussion
This study demonstrates that a strategy of continuing, rather than holding, clinically prescribed long-acting glucose-lowering medications in patients with DM undergoing coronary angiography with possible PCI results in improved peri-procedural glycemic control. This is important since this strategy is not often implemented (9). Alternative strategies of lowering blood glucose at the time of PCI are difficult to implement and not widely used in cardiac catheterization laboratories. Most PCI procedures occur ad-hoc, thus a strategy of continuing long-acting glucose-lowering medications prior to coronary angiography in all patients who may undergo PCI appears reasonable.
The impact of intensive glycemic control on macrovascular disease remains controversial. Long-term follow-up in early trials demonstrated that relatively short periods of intensive glycemic control was associated with a reduction in the incidence of myocardial infarction (MI) and cardiovascular death in patients with Type 1 and Type 2 DM (14–15). In contrast, more recent studies demonstrated either no effect or a detrimental effect of long-term intensive glycemic control on cardiovascular outcomes (16–19). While The Action to Control Cardiovascular Risk in Diabetes study showed that maintenance of tight long-term glycemic control in patients with DM was associated with higher mortality, rates of non-fatal MI were significantly 10% lower in the intensive glycemic control arm (16). Given these findings in the chronic setting, the study of targeted glycemic control in acute settings where patients with DM are at risk for MI, such as PCI, is warranted.
Lending further support to the potential benefit of glycemic control in the peri-PCI setting is the Randomized Trial of Insulin-Glucose Infusion followed by Subcutaneous Insulin treatment in Diabetic Patients with Acute MI (DIGAMI) study, which demonstrated that higher admission glucose predicted higher mortality, and that with intensive insulin treatment, glucose was lowered and long-term mortality decreased (6). It was uncertain, though, whether the inpatient or outpatient intervention was responsible for the reduction in mortality. The DIGAMI-2 trial confirmed that glucose was an independent predictor of mortality, but no difference in mortality was noted between the intensive and conventional treatment groups (7). However, the study reached less than half of its sample size goal and did not reach the target glucose of 90 to 126mg/dL in the intensive treatment arm. Multiple trials attempting to demonstrate a benefit with glucose-insulin-potassium infusions have yielded conflicting results, possibly due to the failure to reach target glucose levels in the treatment arm. Finally, a recent study by Marefella et al, demonstrated optimal peri-procedural glycemic control in the setting of ST-segment elevation MI to reduce oxidative stress, inflammation, and in-stent restenosis on 6-month follow-up coronary angiogram (20). While these trials were all conducted in patients with acute MI, the benefits of glycemic control may extend to the almost 35% of patients that go on to develop peri-procedural myonecrosis and possibly even more so those with clinically significant peri-procedural MI.
Many physicians believe that improved control of blood glucose in the catheterization laboratory is likely to improve peri-procedural outcomes but are avoiding treatment of glucose due to concern for hypoglycemia (9). Our current study should allay those concerns for most patients provided that appropriate precautions are taken. Although there were no serious adverse events in our study, we did observe 2 hypoglycemic events in the Continue group. Both of these patients were on both full dose insulin and a sulfonylurea agent. Since hypoglycemia is more common with sulfonylureas than with other oral glucose-lowering agents, caution should be taken in continuing the combination of sulfonylureas and full dose insulin prior to procedure (21). Clinicians should note that biguanides, which are not associated with hypoglycemia, are now recommended as first-line medications, and sulfonylurea use is declining (22).
Increased platelet activity is seen in patients with inadequately controlled DM (23–24). While light transmission aggregometry is historically considered to be the gold standard of measuring platelet activity, this technique is time-consuming, requires large samples, and yields variable results based on technique. We measured multiple convenient, but well studied, markers of acute platelet activation. Since activated platelets degranulate and rapidly bind to monocytes and leukocytes, MPA and LPA are markers of acute platelet adhesion and activation and shown to circulate in patients with CAD (25–27). Circulating activated degranulated platelets rapidly shed surface p-selectin, resulting in an increase in plasma levels, and soluble p-selectin correlates with other markers of platelet activity and is associated with cardiovascular events (28). In the current study, patients in the Hold group had greater platelet activity compared to the Continue and no DM groups. Given the association between elevated platelet activity and clinical outcomes after PCI, the data suggest a possible mechanism of benefit with continuing long-acting glucose-lowering medications prior to PCI in patients with DM (29). For example, increased platelet activity may contribute to acute peri-procedural stent thrombosis. Acute stent thrombosis occurs in up to 2% of patients undergoing PCI, even in the setting of contemporary antiplatelet and anticoagulant therapy, and is associated with poor outcomes (30).
There are several limitations to this study. First, this was a single center study with a study population consisting almost entirely of men due to recruitment from the Veterans Affairs healthcare system. Second, the endpoints studied are surrogates for clinical outcomes. Third, the study is underpowered to determine the safety of continuing long-acting glucose-lowering medications in longer PCI procedures. Less than 1/3 of our cohort underwent PCI, and procedure times, as well as doses of sedating medications, can be greater with PCI compared with coronary angiography alone. However, given that the majority of PCI procedures occur ad-hoc after coronary angiography, implementing a strategy of routinely continuing long-acting glucose-lowering medications in patients with DM would require that it be implemented in all patients referred for coronary angiograph with the possibility of PCI. Despite these limitations, this is a randomized clinical trial and the only study to evaluate this novel strategy of continuing all clinically prescribed long-acting glucose-lowering medications to maintain peri-procedural euglycemia in patients undergoing coronary angiography with possible PCI.
Acknowledgements
We acknowledge the contributions of Jonathan Willner, MD, Vanessa Valdes, Gregory Rosner, and Candace Tannis of New York University School of Medicine to the data collection.
Dr. Schwartzbard is a speaker for Takeda Pharmaceuticals. Drs. Shah and Sedlis receive research support from Takeda Pharmaceuticals; this relationship did not exist during the design, conduct and data analysis of the presented study.
Funding Sources
Dr. Shah was partially funded by the American College of Cardiology Foundation/Merck Research Fellowship (2011–2012) and by an NIH/NHLBI grant (T32HL098129) (2012–2013).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Shah B, Liou M, Grossi E, Mass H, Lorin JD, Danoff A, Sedlis SP. Relation of elevated periprocedural blood glucose to long-term survival after percutaneous coronary intervention. Am J Cardiol. 2005;96:543–546. doi: 10.1016/j.amjcard.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Robertson BJ, Gascho JA, Gabbay RA, McNulty PH. Usefulness of hyperglycemia in predicting renal and myocardial injury in patients with diabetes mellitus undergoing percutaneous coronary intervention. Am J Cardiol. 2004;94:1027–1029. doi: 10.1016/j.amjcard.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 3.Corpus RA, George PB, House JA, Dixon SR, Dixon SR, Ajluni SC, Devlin WH, Timmis GC, Balasubramaniam M, O'Neill WW. Optimal glycemic control is associated with a lower rate of target vessel revascularization in treated type II diabetic patients undergoing elective percutaneous coronary intervention. J Am Coll Cardiol. 2004;43:8–14. doi: 10.1016/j.jacc.2003.06.019. [DOI] [PubMed] [Google Scholar]
- 4.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent vasodilation and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetolgia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 5.Yazici M, Demircan S, Durna K, Yasar E, Acar Z, Sahin M. Effect of glucose-insulin-potassium infusion on myocardial damage due to percutaneous coronary revascularization. Am J Cardiol. 2005;96:1517–1520. doi: 10.1016/j.amjcard.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 6.Malmberg K, Norhannar A, Wedel H, Rydén L. Glycometabolic state at admission: Important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626–2632. doi: 10.1161/01.cir.99.20.2626. [DOI] [PubMed] [Google Scholar]
- 7.Malmberg K, Ryden L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J, Hildebrandt P, MacLeod K, Laakso M, Torp-Pedersen C, Waldenström A. DIGAMI 2 Investigators. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26:650–661. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 8.Mehta SR, Yusuf S, Díaz R, Zhu J, Pais P, Xavier D, Paolasso E, Ahmed R, Xie C, Kazmi K, Tai J, Orlandini A, Pogue J, Liu L Create-ECLA Trial Group Investigators. Effect of glucose-insulin- potassium infusion on mortality in patients with acute st-segment elevation myocardial infarction; The CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 9.Shah B, Danoff A, Radford MJ, Rolnitzky L, Sedlis SP. Periprocedural management of the diabetic patient undergoing coronary angiography: Current practice. Arch Intern Med. 2012;172:1514–1516. doi: 10.1001/archinternmed.2012.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah B, Sha D, Xie D, Mohler ER, 3rd, Berger JS. The relationship between diabetes mellitus, metabolic syndrome and platelet activity as measured by mean platelet volume, the National Health and Nutrition Examination Survey, 1999–2004. Diabetes Care. 2012;35:1074–1078. doi: 10.2337/dc11-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blocked Randomization with Randomly Selected Block Sizes. Int J Environ Res Public Health. 2011;8:15–20. doi: 10.3390/ijerph8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 13.Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R. A prospective randomized multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35:1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 14.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 15.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 16.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The ADVANCE Collaborative Group. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 18.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S. ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 20.Marfella R, Sasso FC, Siniscalchi M, Paolisso P, Rizzo MR, Ferraro F, Stabile E, Sorropago G, Calabrò P, Carbonara O, Cinquegrana G, Piscione F, Ruocco A, D'Andrea D, Rapacciuolo A, Petronella P, Bresciani A, Rubino P, Mauro C, Paolisso G. Peri-procedural tight glycemic control during early percutaneous coronary intervention is associated with a lower rate of in-stent restenosis in patients with acute ST-elevation myocardial infarction. J Clin Endocrinol Metab. 2012;97:2862–2871. doi: 10.1210/jc.2012-1364. [DOI] [PubMed] [Google Scholar]
- 21.Guthrie RM. Evolving Therapeutic Options for Type 2 Diabetes Mellitus: An Overview. Postgraduate Medicine. 2012;124:82–89. doi: 10.3810/pgm.2012.11.2614. [DOI] [PubMed] [Google Scholar]
- 22.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 24.Davi G, Catalan I, Averna M. Thromboxane biosynthesis and platelet function in type 2 DM. N Engl J Med. 1990;32:1769–1774. doi: 10.1056/NEJM199006213222503. [DOI] [PubMed] [Google Scholar]
- 25.Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, Hechtman HB, Michelson AD. Increased platelet reactivity and circulating monocyte platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol. 1998;31:352–358. doi: 10.1016/s0735-1097(97)00510-x. [DOI] [PubMed] [Google Scholar]
- 26.Mickelson JK, Lakkis NM, Villarreal Levy G, Hughes BJ, Smith CW. Leukocyte activation with platelet adhesion after coronary angioplasty: a mechanism for recurrent disease? J Am Coll Cardiol. 1996;28:345–353. doi: 10.1016/0735-1097(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 27.Freedman JE, Loscalzo J. Platelet monocyte aggregates: bridging thrombosis and inflammation. Circulation. 2002;105:2130–2132. doi: 10.1161/01.cir.0000017140.26466.f5. [DOI] [PubMed] [Google Scholar]
- 28.Ferroni P, Martini F, Riondino S, La Farina F, Magnapera A, Ciatti F, Guadagni F. Soluble P-selectin as a marker of in vivo platelet activation. Clin Chim Acta. 2009;399:88–91. doi: 10.1016/j.cca.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Kabbani SS, Watkins MW, Ashikaga T, Terrien EF, Holoch PA, Sobel BE, Schneider DJ. Platelet reactivity characterized prospectively: a determinant of outcome 90 days after percutaneous coronary intervention. Circulation. 2001;104:181–186. doi: 10.1161/01.cir.104.2.181. [DOI] [PubMed] [Google Scholar]
- 30.Dangas GD, Claessen BE, Mehran R, Brener S, Brodie BR, Dudek D, Witzenbichler B, Peruga JZ, Guagliumi G, Moses JW, Lansky AJ, Xu K, Stone GW. Clinical outcomes following stent thrombosis occurring in-hospital versus out-of-hospital. J Am Coll Cardiol. 2012;59:1752–1759. doi: 10.1016/j.jacc.2011.12.042. [DOI] [PubMed] [Google Scholar]