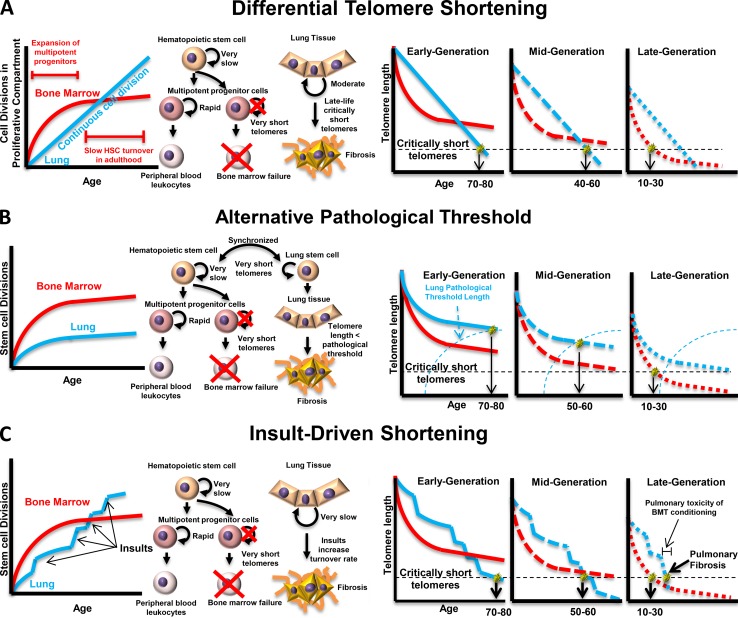
Figure 2.
Three models for the genetic anticipation observed in telomeropathies. Models that explain the anticipation observed in impaired telomere maintenance rely on different assumptions about the behavior of organ-specific stem cells. (A) If lung tissue stem cells have a constant cell division rate that is slower than hematopoietic stem cells during expansion of the multipotent progenitor (MPP) cell compartment but slower than the maintenance HSC division rate after the MPP pool is established, a point will exist in middle age when lung telomere lengths are shorter than MPP/HSC telomere lengths; if the patient started with an initial telomere length sufficient to avoid critically short telomeres until after this point, the patient will experience IPF, whereas patients with initial telomere lengths short enough that they encounter the critical length before that point will experience bone marrow failure. (B) If stem telomere attrition rates are synchronized after development, lung tissue must be subject to an alternative pathological threshold for telomere length that may be age dependent. In this case, patients with longer initial telomere lengths will never encounter critically short telomeres in their bone marrow, while their lung tissue will encounter the alternative threshold, leading to IPF. Patients with shorter initial telomere lengths will encounter critically short telomeres in their bone marrow before the critical threshold for the lungs, leading to bone marrow failure. (C) If telomere attrition rates are synchronous under normal conditions but lung tissue is differentially sensitive to environmental insults, an alternative pathological threshold is not necessary to explain the anticipation. Patients with longer initial telomeres will eventually be driven into IPF by environmental insults while never encountering the critically short telomere length in the HSC compartment, whereas patients with shorter initial telomere length will encounter the critical threshold before insult-driven lung failure. This model is further supported by observations of pulmonary fibrosis after pulmonary toxicity from bone marrow transplant conditioning.