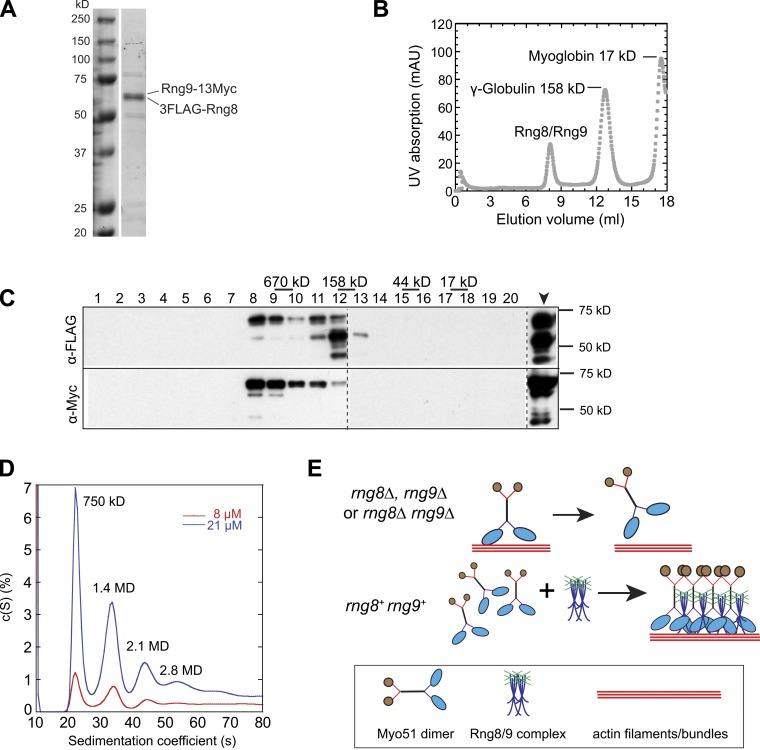
Figure 9.
Rng8 and Rng9 form higher-order complexes. (A) SDS-PAGE of purified 3FLAG-Rng8 and Rng9-13Myc from a myo51Δ strain. (B) Purified Rng8 and Rng9 form a large protein complex revealed by gel filtration. Elution of the purified Rng8–Rng9 complex mixed with standard proteins from a calibrated gel filtration column is shown. The peaks of two standard proteins are indicated. (C) Western blotting using anti-FLAG or anti-Myc antibodies to detect Rng8 or Rng9 from the elution fractions 1–20 (1 ml/fraction) and the sample before gel filtration (arrowhead). Broken lines indicate that intervening lanes have been spliced out. (D) Distribution of sedimentation coefficients of the Rng8–Rng9 complex at two protein concentrations, assuming a continuous c(S) distribution. Molecular weight for individual peaks are indicated. (E) Possible mechanism for the regulation of Myo51 by the Rng8–Rng9 complex in vivo. (top) Myo51 forms dimers without Rng8 and/or Rng9, and it easily detaches from actin filaments/bundles due to its low duty ratio (Clayton et al., 2010). (bottom) The Rng8–Rng9 complexes cluster multiple Myo51 dimers, and the motor heads cooperate to maintain the attachment and move continuously on actin filaments/bundles. This motor–track interaction may help to stabilize/organize actin filaments/bundles.