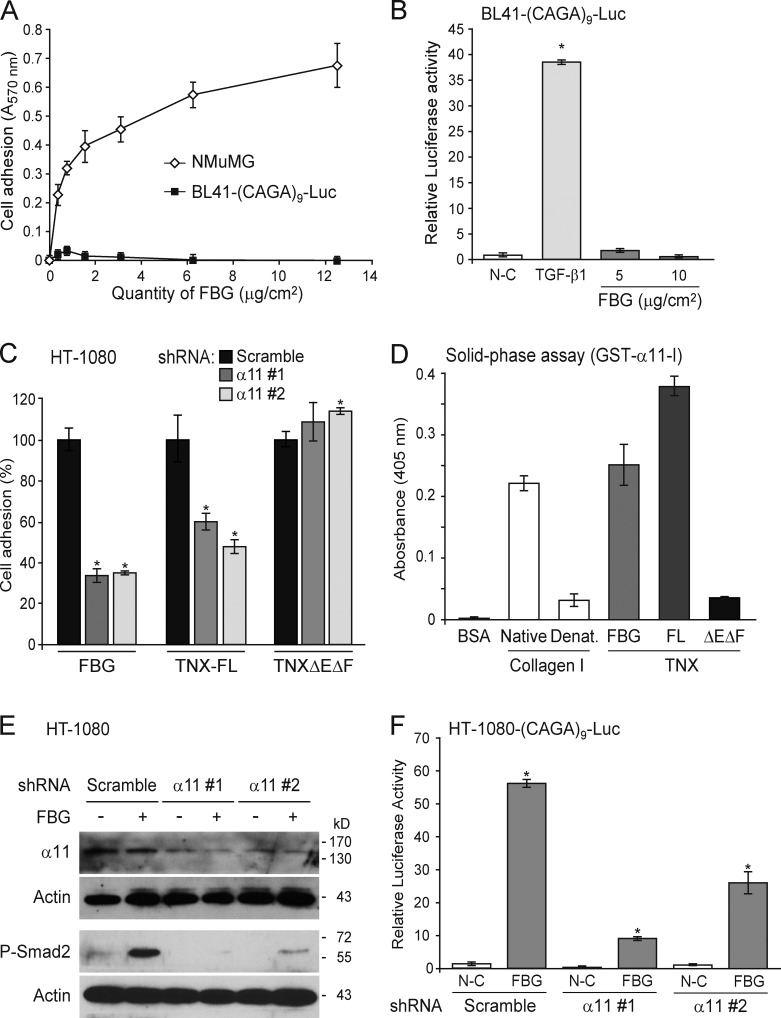
Figure 9.
Activation of latent TGF-β by the FBG domain requires cell adhesion and the α11β1 integrin. (A) Adhesion of NMuMG and BL41-(CAGA)9-Luc cells to an increasing quantity of recombinant FBG domain. (B) Luciferase activity of BL41-(CAGA)9-Luc reporter cells cultured for 24 h on coated FBG domain or treated with 5 ng/ml TGF-β1. (C) Adhesion analysis of HT-1080 fibrosarcoma cells transiently expressing a scrambled shRNA or one of two shRNAs (#1 and #2) targeting different sequences of the ITGA11 mRNA and cultured for 30 min onto coated recombinant FBG domain or other TNX fragments (111 pmol/cm2). Results represent the percentage of cell adhesion relative to the scramble shRNA condition for each recombinant protein analyzed. (D) Solid-phase assay of the interaction between the inserted domain of α11 integrin chain in fusion with GST (1 µM) and 5 µg/ml of native or denatured (Denat.) type I collagen or the different TNX derivatives (111 nM). FL, full length. (E) Immunoblotting analysis of phospho-Smad2 and the α11 integrin chain in HT-1080 cells transiently transfected as in C and seeded for 3 h onto uncoated (−) or 222 pmol/cm2 FBG-coated (+) dishes. (F) Firefly luciferase activity of HT-1080 cells transiently cotransfected with the Smad-responsive (CAGA)9-Luc reporter construct and a scrambled or an ITGA11-targeting shRNA and cultured for 24 h in noncoated (N-C) or 222 pmol/cm2 FBG-coated wells. *, P < 0.05 versus control condition. Error bars are means ± SD.