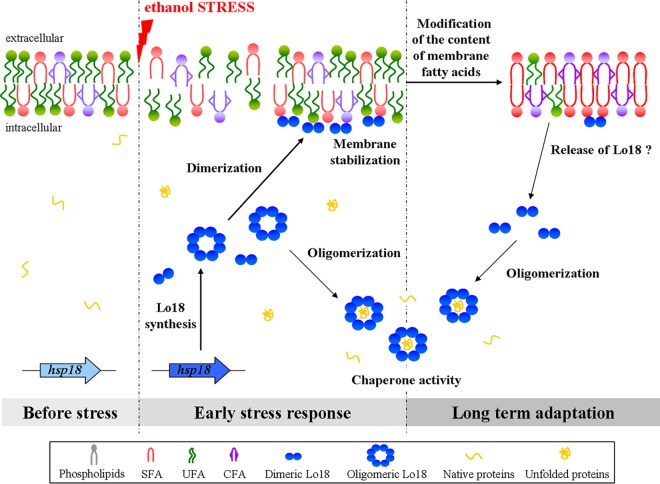
FIG 6.
Model of Lo18 activities in response to ethanol stress in O. oeni cells. Ethanol stress increases membrane fluidity. To maintain membrane integrity, O. oeni produces the sHsp Lo18 that has the dual role of both membrane stabilization and protection of proteins from aggregation. The dissociation of Lo18 oligomers may be crucial for these activities. Dimers are preferentially found at the membrane surface. During stress, the fatty acid content of the bacterial membrane is modified. This leads to the release of dimeric Lo18 from the membrane, making it available for oligomerization and chaperone activity for cytoplasmic proteins. SFA, saturated fatty acids; UFA, unsaturated fatty acids.