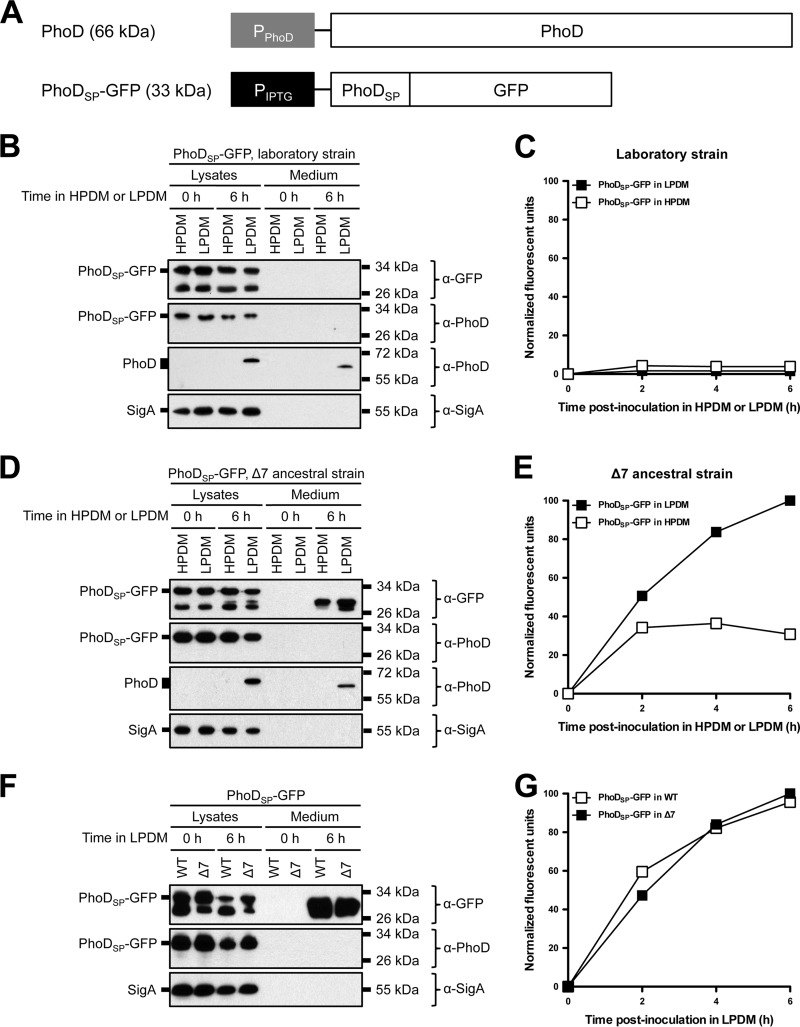
FIG 2.
Protein secretion from laboratory, Δ7 ancestral, and wild-type ancestral strains of B. subtilis under phosphate-limiting conditions. (A) Endogenous PhoD gene arrangement and PhoDSP-GFP construct design. The predicted molecular mass of each protein is indicated in parentheses. (B and D) Expression and secretion of total GFP from laboratory (B) and Δ7 ancestral strains (D). The indicated strains expressing PhoDSP-GFP were grown overnight in HPDM supplemented with 1 mM IPTG. The next day, the cells were washed and resuspended in either HPDM or LPDM supplemented with 1 mM IPTG. After 0 and 6 h in LPDM, the cells were lysed and the medium was precipitated with TCA. The cell lysates and precipitated medium were examined by Western blot probing with α-GFP, α-PhoD, and α-SigA antibodies. The migration of PhoDSP-GFP, PhoD, and SigA bands is indicated to the left of each blot. The migration of molecular mass standards is indicated to the right of each blot. (C and E) Secretion of folded, fluorescent GFP from laboratory and Δ7 ancestral strains. The indicated strains expressing PhoDSP-GFP were grown overnight as described for panels B and D. At the indicated time points, the medium was harvested and GFP fluorescence was measured. Relative fluorescent units were normalized to account for differences in cell density. Results from one representative experiment are shown. (F and G) Expression and secretion of total GFP (F) and secretion of fluorescent GFP (G) from wild-type (WT) ancestral and Δ7 ancestral strains. The indicated strains were grown and the samples were prepared as described for panels B through E. Relative fluorescent units were normalized to account for differences in cell density. Results from one representative experiment are shown.