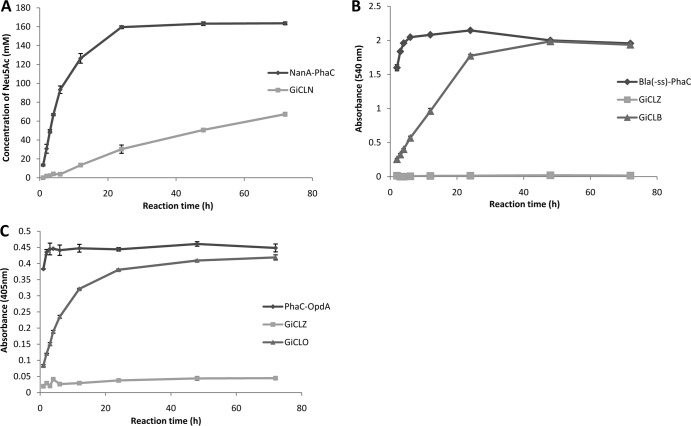
FIG 5.
Reaction time courses for all enzymes immobilized to GFP particles compared to the same enzyme immobilized to PHA granules. Each reaction proceeded for 72 h with samples taken at 1 h, 2 h, 3 h, 4 h, 6 h, 12 h, 24 h, 48 h, and 72 h. (A) Twenty-five micrograms of NanA-PhaC or GiCLN fusion protein reacted with 0.2 M N-acetyl-d-mannosamine and 1 M sodium pyruvate at 50°C. The production of N-acetyl neuraminic acid was quantified by HPLC. (B) Fifty micrograms of Bla(−ss)-PhaC, GiCLZ, or GiCLB fusion protein reacted with 1% starch solution at 25°C. The release of maltose was quantified by the α-amylase colorimetric assay. (C) Fifty micrograms of PhaC-OpdA, GiCLZ, or GiCLO fusion protein reacted with 200 μM methyl parathion at 25°C. The release of paranitrophenol was quantified by spectroscopy. Error bars are ±1 standard deviation (n = 3). G, GFP; iC, inactive PhaC; L, linker; N, NanA; B, BLA; O, OpdA; Z, ZZ.