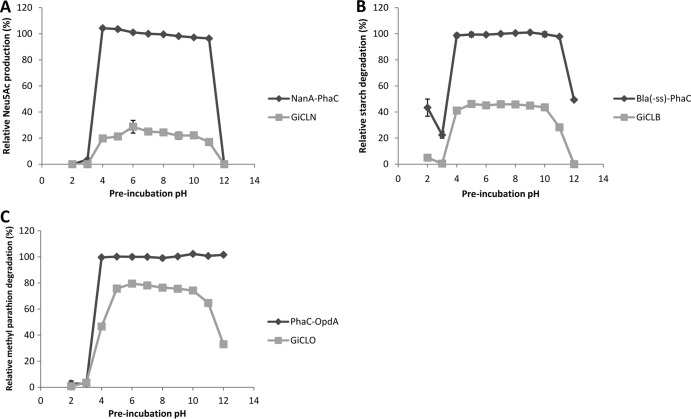
FIG 8.
The pH stability of enzymes immobilized to GFP particles. The particles were preincubated at the indicated pH (2 to 12) for 10 min and then subjected to their respective reactions. Activity is relative to the PhaC bead at pH 7.0. (A) Twenty-five micrograms of NanA-PhaC or GiCLN fusion protein reacted with 0.2 M N-acetyl-d-mannosamine and 1 M sodium pyruvate at 50°C. The production of N-acetyl neuraminic acid was quantified by HPLC. (B) Fifty micrograms of Bla(−ss)-PhaC or GiCLB fusion protein reacted with 1% starch solution at 25°C. The release of maltose was quantified by the α-amylase colorimetric assay. (C) Fifty micrograms of PhaC-OpdA or GiCLO fusion protein reacted with 200 μM methyl parathion at 25°C. The release of paranitrophenol was quantified by spectroscopy. Error bars are ±1 standard deviation (n = 3). G, GFP; iC, inactive PhaC; L, linker; N, NanA; B, BLA; O, OpdA.