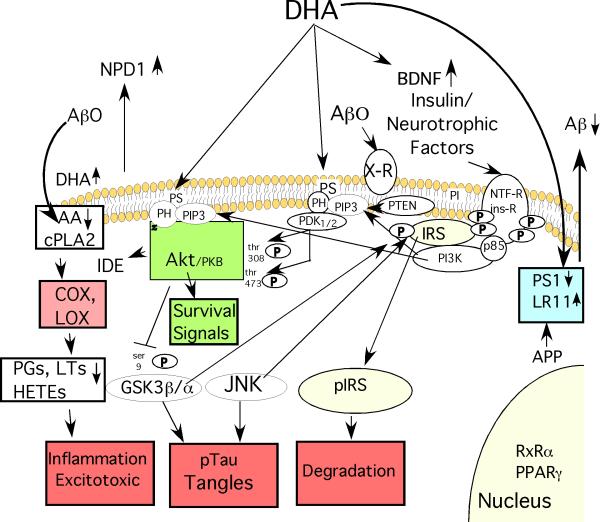
Fig. 1. Some of DHA's Protective Effects against Alzheimer Disease ( AD).
DHA reduces the production of the β-amyloid peptides (Aβ) from amyloid precursor protein (APP) by increasing expression of the anti-amyloidogenic chaperone LR11, a protein which prevents APP from reaching the proteolytic secretases that cut Aβ from APP. DHA can also reduce expression of presenilin 1 (PS1), a critical component of the gamma secretase that creates Aβ. Reducing Aβ production lowers levels of Aβ oligomers (Aβ0) that act on candidate membrane receptors (X-R) with multiple deleterious signal transduction effects. By incorporating into membrane phospholipids, DHA may also have multiple “fluidizing” effects on membrane structure, lipid raft and protein-protein coupling discussed in the text. DHA incorporation will also competitively reduce membrane levels of arachidonic acid (AA), whose release from membrane phospholipids by phospholipase A2 (PLA2) generates free intracellular AA that is a substrate for cyclooxygenase (COX) and lipoxygenase (LOX) enzymes that produce various prostaglandin (PG), Hete and leukotriene (LT) products known to promote inflammation and excitotoxicity that are elevated in AD pathogenesis. Aβ0 activate inflammation in glia and neuronal cPLA2 resulting in elevated AA and its COX and LOX products in AD and APP transgenic mice. DHA not only reduces these products but DHA itself is transformed by LOX to a potent neuroprotective mediator, neuroprotectin D1 (NPD1) and related mediators which have anti-inflammatory, neuroprotective and other anti-AD activities. DHA can activate PPAR and RxR transcription factors. DHA has also been reported to be rate limiting for phosphatidylserine on the inner membrane leaflet which promotes docking with the PI3-K product PIP-3 and resultant activation of both Akt and upstream PDK, the critical effectors of PI3-K /PDK/ Akt survival signaling pathway stemming from activation of insulin and neurotrophic factor receptors. Signaling through this PI3-K pathway upregulates an Aβ protease insulin degrading enzyme (IDE), has multiple pro-survival effects on apoptotic regulators (“survival signals”) that protect neurons and also inhibits a major tau kinase, GSK3β. Through this or other actions DHA also reduces activity of another important tau kinase, JNK. GSK3β and JNK are known to hyperphosphorylate tau resulting in neurofibrillary tangle formation, but they also target the critical insulin receptor substrates (IRS-1 and 2). This causes IRS to uncouple from both insulin and neurotrophic factor receptors and be rapidly degraded resulting in a state of insulin/neurotrophin resistance. This is likely to contribute to synaptic loss and a dying back of neuritic arbor. Finally, DHA can increase production of brain derived neurotrophic factor (BDNF), a factor, which plays an important positive role in activity-dependent synaptic plasticity, but is lost in AD. In summary, DHA has multiple pleiotropic activities predicted to slow AD pathogenesis at many levels.