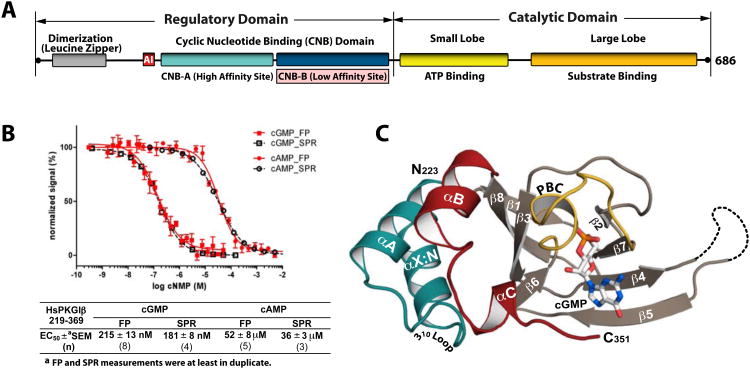
Figure 1. Domain organization and overall structure of the PKG Iβ (219-369):cGMP complex.
(A) Domain organization of PKG Iβ with CNB-B highlighted. AI: autoinhibitor sequence. (B) Affinity measurements of PKG Iβ (219-369) for cGMP and cAMP The construct has a 240-fold selectivity for cGMP. Competition FP curves are shown in red and competition SPR curves are shown in black. Error bars denote standard error of measurement. (C) Overall structure of the PKG Iβ 219-369:cGMP complex with the secondary structure elements labeled. The phosphate binding cassette (PBC) is colored yellow, the αB and αC helices red, the N-terminal helices teal, and cGMP is colored by atom type. The N- and C-termini are labeled with their corresponding residue number seen in the final model. The disordered β4-β5 loop in the PKG Iβ (219-369):cGMP complex is shown as a dotted line. All structure images were generated using PyMOL (Delano Scientific).