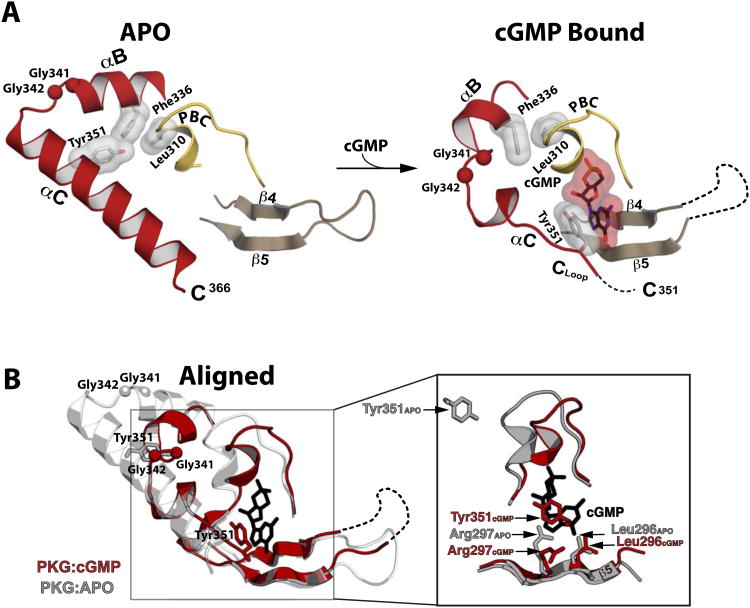
Figure 3. Structural comparison between the apo- and cGMP bound PKG Iβ (219-369).
(A) Regions mediating cGMP interactions are shown. The disordered β4-β5 loop and disordered portion of the αC helix are indicated with a dotted line. The Cα atoms of the Gly-Gly hinge residues (Gly341 and Gly342) are shown as balls. Leu310, Phe336, Tyr351 and cGMP are shown as sticks with transparent surface. (B) The apo (gray) and PKG Iβ 219-369:cGMP (red) structures aligned at the β barrel region. An enhanced view of the binding pocket highlighting the β5 and capping residue interactions with cGMP (black sticks) is shown on the right. In the apo structure, the side chain of Arg297 is extended in a conformation partially occupying the empty cGMP binding pocket.