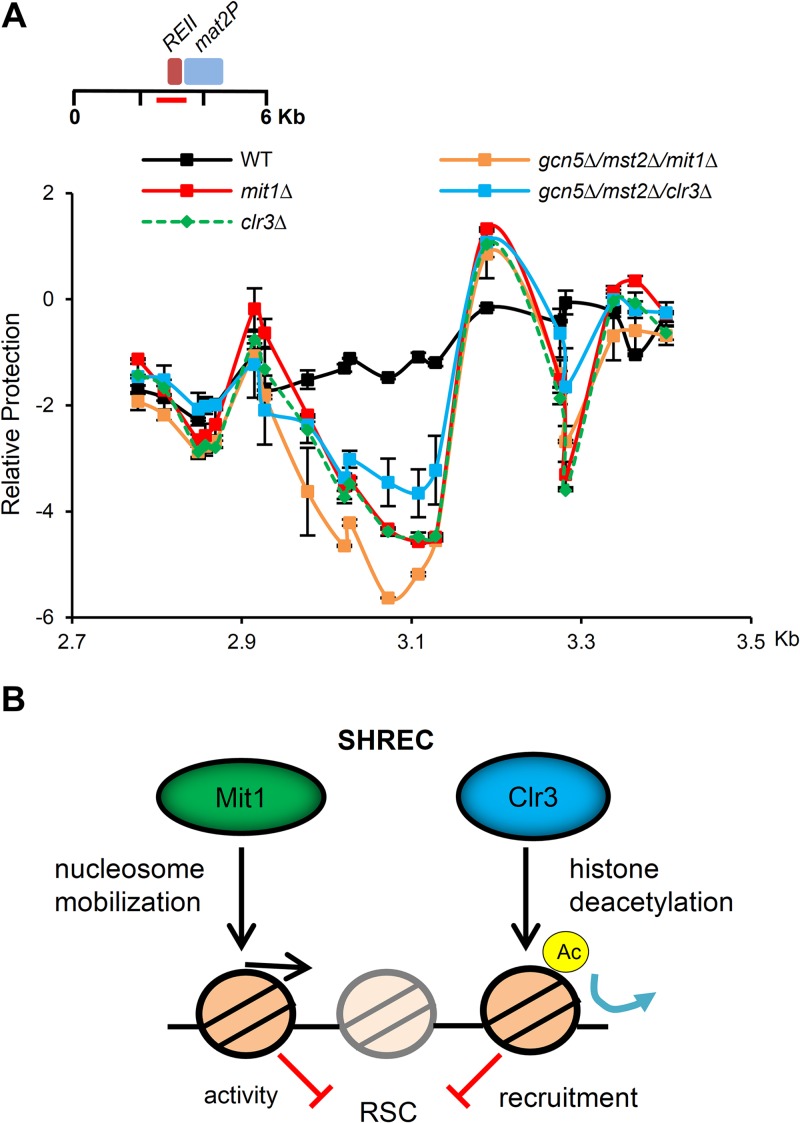
FIG 7.
Prevention of H3K14 acetylation is not sufficient to rescue nucleosome occupancy changes observed in SHREC-deficient cells. (A) Histone acetyltransferase mutant strains cannot suppress NFR formation at REII in SHRECΔ cells. The nucleosome scanning experiment was performed as for Fig. 5E, comparing wild-type cells to clr3Δ and mit1Δ cells as well as the gcn5Δ mst2Δ clr3Δ and gcn5Δ mst2Δ mit1Δ triple deletion mutants. Data are plotted on a log2 scale, error bars represent SEM (n = 2). We note that the occupancy profiles for mit1Δ and clr3Δ are largely overlapping. (B) Model for SHREC contribution to nucleosome occupancy at regions of heterochromatin. We propose a model where SHREC, as well as other chromatin modifiers, such as Set2/Clr6, Spt6, and Asf1/HIRA, prevent localized transient and steady-state nucleosome depletion in heterochromatic domains. SHREC performs this function using distinct but likely related activities in nucleosome mobilization and histone deacetylation in part to oppose the activity of the RSC complex (20). Elimination of NFRs is then important for efficient transcriptional silencing by restricting access to Pol II in regions of heterochromatin.