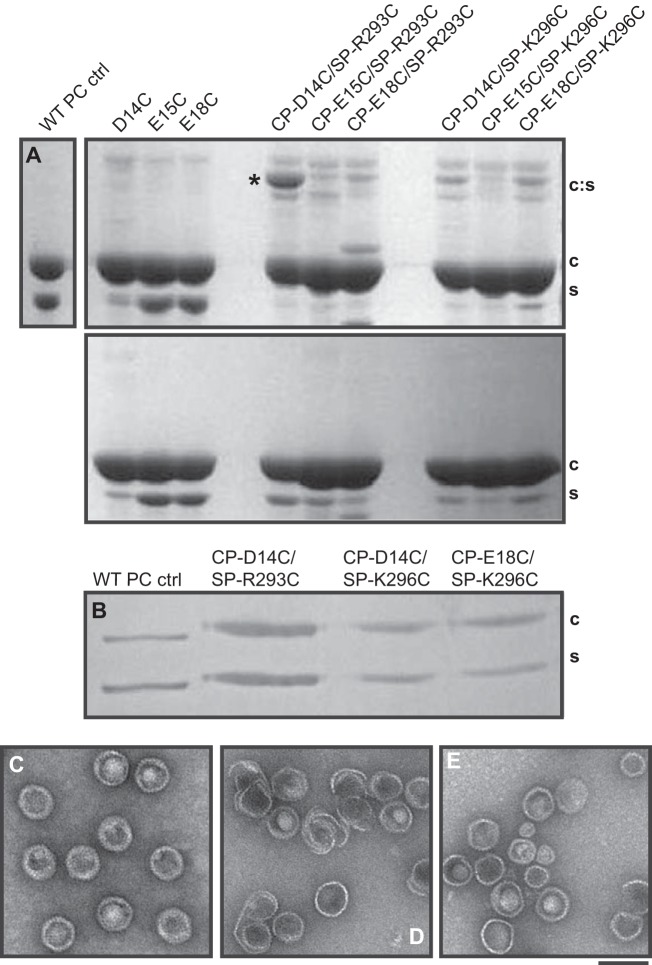
FIG 6.
The coat protein D14C mutant binds strongly to the scaffolding protein R293C mutant and not to other N-arm residues in procapsids. (A) SDS-PAGE analysis of procapsids of CP-D14C, CP-E15C, CP-E18C, CP-D14C/SP-R293C, CP-E15C/SP-R293C, CP-E18C/SP-R293C, CP-D14C/SP-K296C, CP-E15C/SP-K296C, and CP-E18C/SP-K296C cysteine mutants. An intense band at ∼80 kDa was observed for the CP-D14C/SP-R293C double mutant, indicated by the asterisk. This band was not as pronounced for any of the other five double-cysteine mutants. (B) Two-dimensional reducing SDS-PAGE gel for the CP-D14C/SP-R293C, CP-D14C/SP-K296C, and CP-E18C/SP-K296C ∼80-kDa bands. (C to E) Electron micrographs of negatively stained CP-D14C/SP-R293C (C), CP-E15C/SP-R293C (D), and CP-E18C/SP-R293C (E) particles. The bar represents 100 nm.