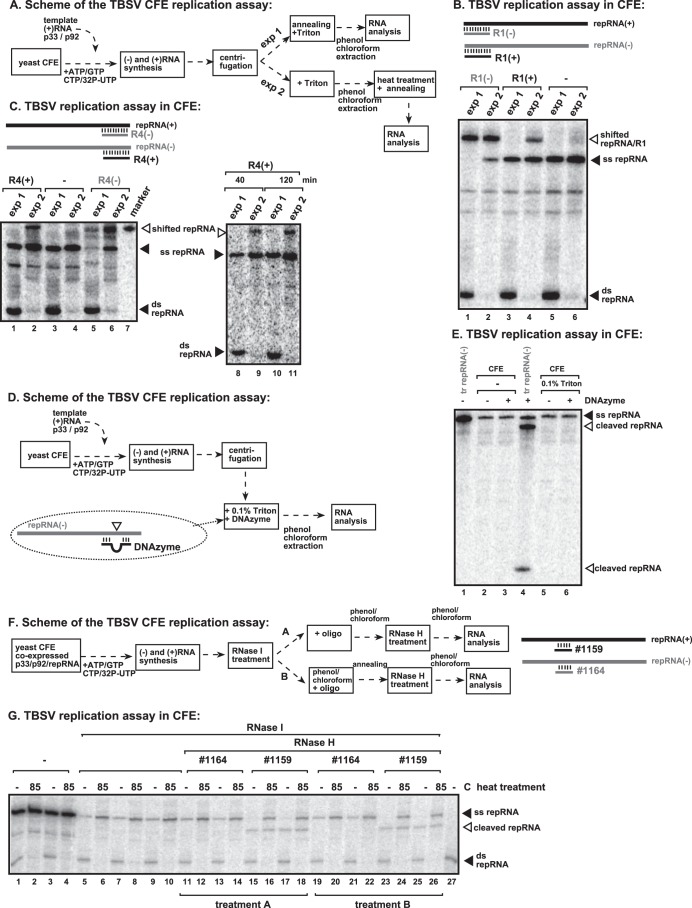
FIG 3.
Lack of free TBSV (−)repRNA among the TBSV RNAs produced in the CFE assay. (A) Scheme of the CFE-based TBSV replication assay. In experiment 1, at the end of the replication assay, we added unlabeled short complementary RNAs to the membrane fraction of the CFE assay mixture in the presence of 0.1% Triton X-100 prior to phenol-chloroform extraction and RNA analysis. In experiment 2, at the end of the replication assay, we added 0.1% Triton X-100 to the membrane fraction of the CFE assay mixture, performed phenol-chloroform extraction, heat denatured the RNAs, and then added unlabeled short complementary RNAs. Note that experiment 2 tests the ability of the short complementary RNAs to specifically anneal to the target RNA in the assay mixture. (B, top) Scheme of the annealed unlabeled short complementary RNAs to the 32P-labeled repRNA products. Note that the annealed RNA duplex changes the migration of the RNA in PAGE gels. (Bottom) Representative PAGE analysis of 32P-labeled repRNA products synthesized by the tombusvirus replicase in the CFE assay. The positions of shifted repRNAs (due to annealing to short complementary RNAs), single-stranded repRNAs, and double-stranded repRNAs are shown. Each experiment was repeated three times. (C) Same assay as in panel B except with a different set of short complementary RNAs. (D) Scheme of the DNAzyme-based cleavage of the 32P-labeled repRNA products. The DNAzyme and 0.1% Triton X-100 were added to the membrane fraction of the CFE assay mixture prior to phenol-chloroform extraction. Note that the DNAzyme must anneal to the free (−)repRNA to induce cleavage of the target (−)repRNA, as shown. (E) Representative denaturing PAGE analysis of the DNAzyme-treated 32P-labeled repRNA products synthesized by the tombusvirus replicase in the CFE assay. Lane 4 shows the DNAzyme cleavage products of the in vitro-transcribed DI-72 (−)repRNA as a positive control that demonstrates the functionality of the DNAzyme. (F) Identification of the polarity of ssRNA present in the membrane-bound VRCs. Shown is a scheme of the CFE-based TBSV replication assay. The CFE was prepared from BY4741 yeast cells expressing tombusvirus p33 and p92pol replication proteins and the TBSV DI-72 (+)repRNA. The RNase I treatment (in the absence of detergent) at the end of the CFE assay removed the released, unprotected, 32P-labeled (+)repRNA, followed by phenol-chloroform extraction. The samples were then annealed to strand-specific DNA oligonucleotides (as shown schematically) and treated with RNase H. (G) PAGE analysis of the 32P-labeled TBSV repRNA products obtained in the CFE assays after RNase H treatment. Note that the RNase I-protected 32P-labeled ssRNA was cleaved by RNase H in the presence of oligonucleotide 1159, demonstrating that the ssRNA represents (+)repRNA in the membrane-bound VRC.