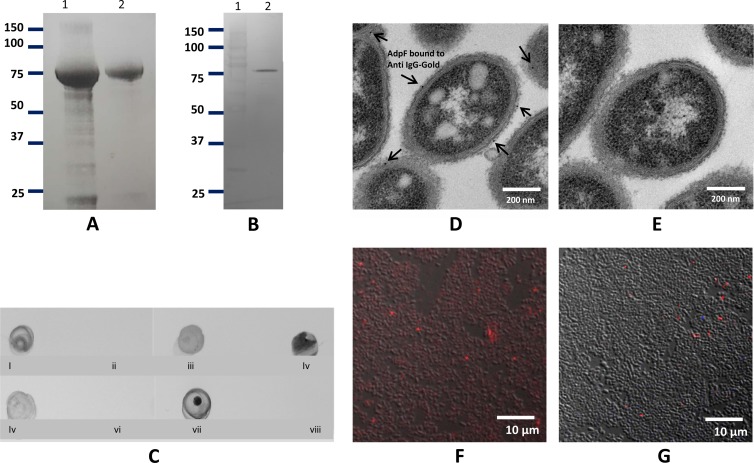
FIG 2.
Purification and cellular localization of AdpF. (A) SDS-PAGE analysis of cell extract after IPTG induction (lane 1) and of purified recombinant AdpF from E. coli (rAdpF) (lane 2). Molecular weight (in thousands) is shown. (B) Western blot analysis of concentrated P. intermedia 17 culture supernatant. Anti-rAdpF antibody was used to identify the protein. Lanes contain molecular weight marker (in thousands) (lane 1) and P. intermedia 17 culture supernatant proteins (lane 2). (C) Dot blot assay. Five-microliter aliquots were spotted on a nitrocellulose membrane and developed with anti-rAdpF antibody. Blots are as follows: P. intermedia 17 cells (i), P. intermedia 17 cells treated with protease (ii), P. intermedia 17 cell lysate (iii), P. intermedia 17 culture supernatant (iv), lysate of E. coli expressing rAdpF (v), lysate of E. coli carrying no insert vector, as a control (vi), purified rAdpF (vii), BSA (viii). (D and E) Surface localization of anti-AdpF antibody on P. intermedia 17 was shown by immunoelectron microscopy. The anti-AdpF antibodies, labeled with gold particle (arrows), were localized in the outer membrane of P. intermedia 17 (D), and there were no detectable anti-AdpF antibodies in P. intermedia 17 after protease treatment (E). (F and G) Localization of Cy5-labeled anti-rAdpF antibody in P. intermedia 17 cells without treatment with protease (F) and following treatment with protease (G). Bacteria binding Cy5-labeled anti-AdpF antibody appear red in the image.