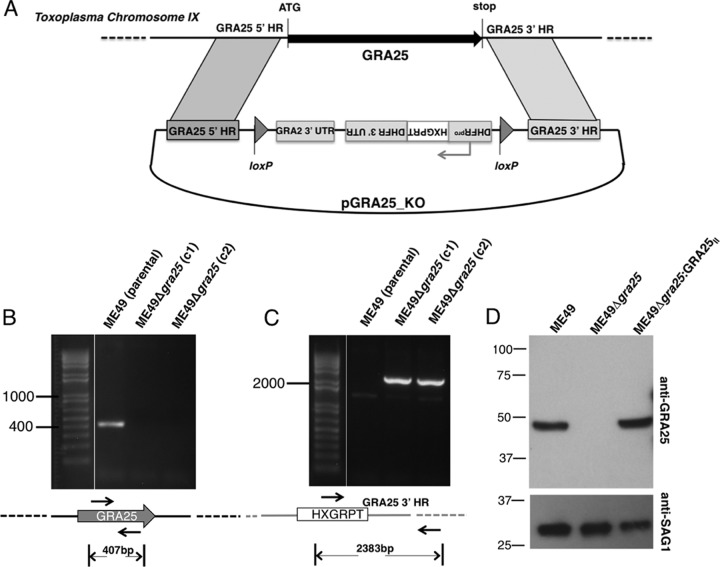
FIG 3.
Generation and complementation of parasites deficient in Toxoplasma GRA25. (A) Schematic of the vector used for the double-crossover event to delete the GRA25 coding region and replace it with an HXGPRT-selectable cassette driven by a DHFR promoter. Homology regions (HR) flanking the GRA25 gene are shown. Bent arrows indicate promoters. loxP sites were used for Cre-mediated excision of the selectable marker in subsequent manipulations. The drawing is not to scale. UTR, untranslated region. (B) Verification of knockout by PCR. Primers were designed within the GRA25 ORF to test for the presence of GRA25 coding sequence in the type II parental strain, ME49, and in two independent ME49Δgra25 knockout clones, c1 and c2. All subsequent experiments were done with clone c1. (C) Primers in the HXGPRT sequence (forward) and in the region downstream of the GRA25 3′ HR used in the deletion construct (reverse) were used to confirm proper integration of the knockout cassette. The expected sizes of the amplification products are shown below the schematics. Relevant marker sizes (in bp) are shown on the left. The drawings are not to scale. (D) Parental (ME49), KO (ME49Δgra25), or complemented (ME49Δgra25:GRA25II) parasite lysates were analyzed by SDS-PAGE. Membranes were probed with polyclonal anti-GRA25 mouse antisera and with anti-SAG1 antibodies (loading control). Migration of size markers is shown on the left (in kilodaltons). Experimental data are representative of the results of at least three independent experiments.