Figure 2.
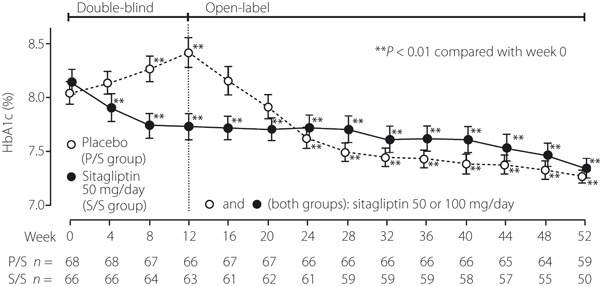
Time course of HbA1c in Japanese patients with type 2 diabetes mellitus. Patients in the P/S group received placebo during the 12‐week double‐blind period and open‐label sitagliptin in the 40‐week open‐label period. Patients in the S/S group received sitagliptin (50 or 100 mg/day) for the subsequent 40 weeks. The data are shown as mean ± SE. In the double‐blind period, the method of last‐observation‐carried‐forward (LOCF) was used to impute values for HbA1c. In the open‐label period, statistics for HbA1c were calculated without LOCF, using at each time‐point the data available for that specific time‐point. The sample sizes at each time‐point are shown beneath the plots. **P < 0.01 compared with week 0.
