Fig. 3.
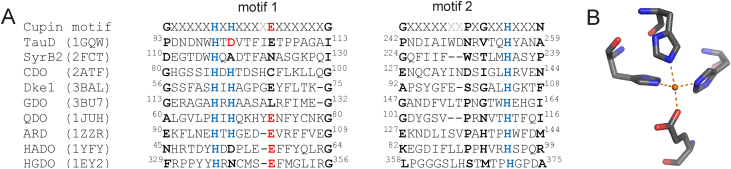
Conserved metal binding motif in cupins. (a.) An alignment of O2 dependent cupins that use Fe(II) as a cofactor is displayed. Metal binding residues are in blue (His) and red (carboxylate), moieties in ‘conserved’ positions are in bold. Variable residues in the cupin motif are in light grey. Abbreviations indicate the MNHEs taurine hydroxylase (TauD), SyrB2, cysteine dioxygenase (CDO), diketone dioxygenase (Dke1), gentisate dioxygenase (GDO), quercetin 2,3-dioxygenase (QDO), aci-reductone dioxygenase (ARD), 3-hydroxyanthranilate3,4-dioxygenase (HADO) and homogentisate dioxygenase (HGDO). The respective protein data bank (PDB) numbers are given in brackets. (b.) The prototypical metal center organization of a cupin-metal center is shown. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
