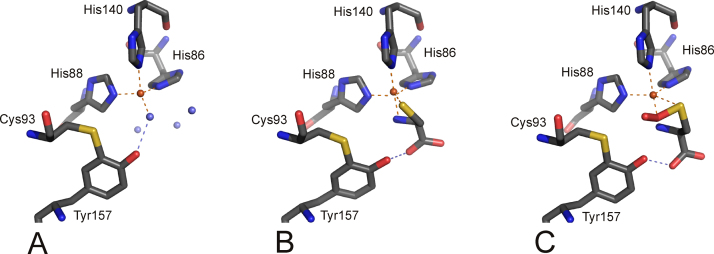
Fig. 8.
Active site of (A) resting CDO (PDB:2B5H), (B) L-cysteine bound CDO (PDB:2IC1) [61] and (C) persulfenate coordinated CDO (PDB:3ELN) [51]. Metal binding amino acids and the covalently linked outer sphere residues Cys93 and Tyr157 are shown. Note that the resting CDO structure contains an Fe(III) ion, while substrate and intermediate bound structures shown have an Fe(II) as active site metal ion. The iron ion is in orange, water molecules are slate blue; oxygen, nitrogen and sulfur-atoms are depicted in red, blue and yellow, respectively. H-bonds are indicated as slate blue dashed lines. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)