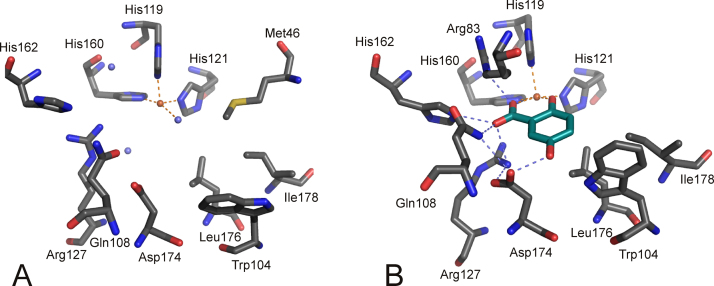
Fig. 19.
Iron center and substrate binding pocket of SDO from P. salicylatoxidans in its (A) free (PDB: 2PHD) and (B) gentisate chelated (PDB: 3NL1) forms. Some residues are omitted for clarity. Oxygen atoms and nitrogen atoms are shown in red and blue, respectively. The substrate ligand is in teal. Water molecules are shown in slate blue. H-bonds are indicated as slate blue dashed lines. Note that substrate binding leads to a movement of the Met46 residue away from the substrate binding site, while Arg83 moves closer above the plane of the substrate ligand. Residues, Gln108, Asp174, His162, Arg127 Trp72 are repositioned due to substrate binding. Further note that residues analogous to Gln108, Asp174, His162 have been suggested to promote acid–base catalysis in GDO. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)