Abstract
Aims/hypothesis
Viruses are candidate causative agents in the pathogenesis of autoimmune (type 1) diabetes. We hypothesised that children with a rapid onset of type 1 diabetes may have been exposed to such agents shortly before the initiation of islet autoimmunity, possibly at high dose, and thus study of these children could help identify viruses involved in the development of autoimmune diabetes.
Methods
We used next-generation sequencing to search for viruses in plasma samples and examined the history of infection and fever in children enrolled in The Environmental Determinants of Diabetes in the Young (TEDDY) study who progressed to type 1 diabetes within 6 months from the appearance of islet autoimmunity, and in matched islet-autoantibody-negative controls.
Results
Viruses were not detected more frequently in plasma from rapid-onset patients than in controls during the period surrounding seroconversion. In addition, infection histories were found to be similar between children with rapid-onset diabetes and control children, although episodes of fever were reported less frequently in children with rapid-onset diabetes.
Conclusions/interpretation
These findings do not support the presence of viraemia around the time of seroconversion in young children with rapid-onset type 1 diabetes.
Keywords: GB virus C, Human herpes virus 6, Human rhinovirus C, Infections, Islet autoimmunity, Next-generation deep sequencing, Parvovirus B19, TEDDY study, Type 1 diabetes mellitus
Introduction
Type 1 diabetes is an autoimmune disorder in which disease onset is preceded by a pre-clinical period of islet autoimmunity [1]. Check points in the pre-clinical pathogenesis include the initiation of islet autoimmunity and the progression to type 1 diabetes; the rate of this process is variable [2]. Although the majority of children who develop type 1 diabetes take years to progress from islet autoimmunity to diabetes, some children have a rapid disease progression in which clinical diabetes occurs within months of the appearance of islet autoantibodies. These patients appear to have uncontrolled beta cell destruction; further investigation of this group may help to identify causative agents of autoimmune diabetes.
Viruses have been frequently, but inconclusively, implicated as candidate agents in the pathogenesis of type 1 diabetes. Although associations between type 1 diabetes and viral infections have been repeatedly reported [3-9], in particular for enterovirus infections, there are also numerous reports that fail to confirm such findings [10-12]. Exposure in children with a rapid progression to clinical diabetes may possibly take place at a higher dose or in a shorter time window before the appearance of islet autoimmunity. We therefore examined the history of infections and fever and analysed plasma samples by unbiased deep sequencing for the presence of viruses in the period around the initiation of islet autoimmunity in children who transitioned within 6 months from autoimmunity to disease in The Environmental Determinants of Diabetes in the Young (TEDDY) study [13].
Methods
TEDDY study
TEDDY is a prospective cohort study with the primary objective of identifying environmental factors associated with increased risk of islet autoimmunity and type 1 diabetes; it includes three centres in the USA (Colorado, Georgia/Florida and Washington) and three in Europe (Finland, Germany and Sweden). Infants younger than 4.5 months and carrying type-1-diabetes-associated HLA alleles (HLA-DR, DQ) were eligible to participate. From 2004 to 2010, TEDDY screened >420,000 newborns and identified 21,589 children with high-risk HLA-DR/DQ genotypes. Of these, 8,677 (932 with first-degree family history of type 1 diabetes and 7,745 without such history) were enrolled in the prospective follow-up. Participants were seen and blood collected every 3 months up to 4 years of age, and every 6 months thereafter. Written informed consent was obtained from the parents. The study was approved by the ethical committees of the participating sites [13].
Study outcome
The study outcome is the appearance of confirmed persistent islet autoimmunity, defined as positive for at least one autoantibody (GAD65A, islet antigen-2 [IA-2A] or insulin autoantibody [IAA]) in both TEDDY core laboratories (Barbara Davis Center, Aurora, CO, USA and the University of Bristol, Bristol, UK) in two consecutive samples or in one sample in children who developed diabetes before a follow-up sample was available for autoantibody testing [14]. Families were notified of the child's autoantibody results at their next study visit. The study endpoint is the development of type 1 diabetes as defined by American Diabetes Association criteria [15].
Study participants and design
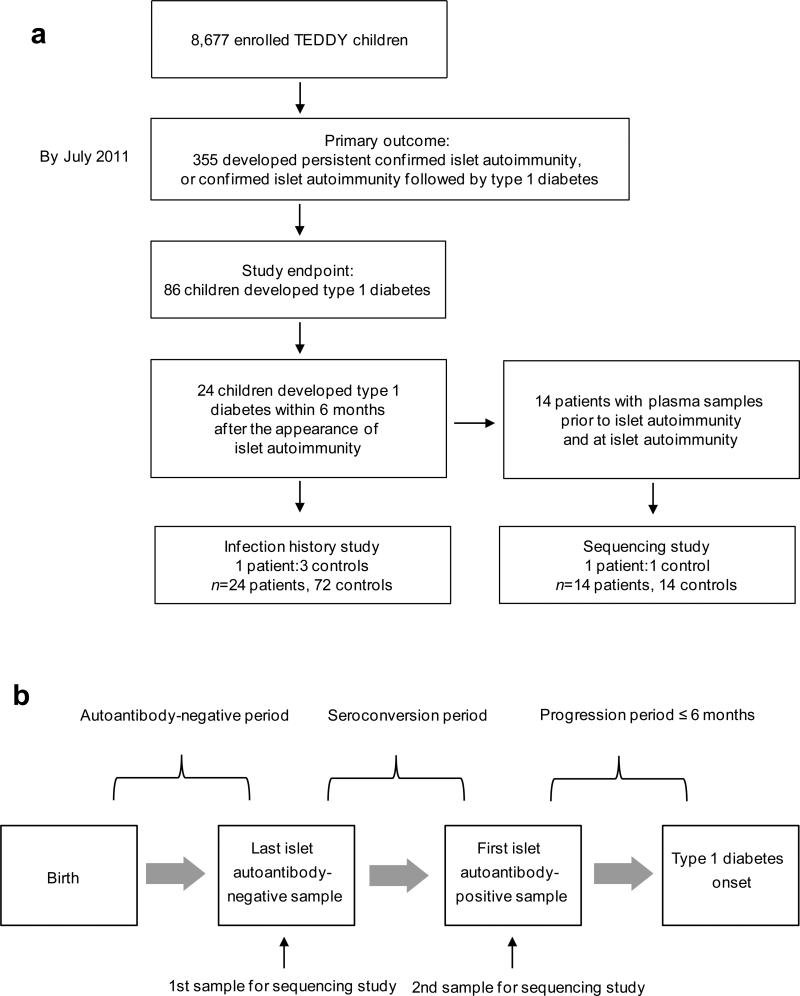
Of the TEDDY participants, 355 had islet autoimmunity, and 86 of these had progressed to type 1 diabetes by July 2011 when the current study was designed. Twenty-four of the children developed type 1 diabetes within 6 months from the appearance of islet autoimmunity and were selected for our study (Fig. 1a). Two nested case–control studies were designed.
Fig. 1.
(a) Flow chart of the study population for the infection history and sequencing studies, and (b) flow chart of the time points investigated in the infection history and sequencing study
Sequencing study
This study investigated whether viral sequences were present in plasma samples at two time points: (1) the ‘last islet-autoantibody-negative sample’; and (2) the first islet-autoantibody-positive ‘seroconversion sample’ (Fig. 1b). Fourteen of the 24 rapid-onset patients had samples with sufficient volume available at both time points. For each of these 14 patients one control child was selected. Controls were children who participated in the TEDDY study but remained negative for all three diabetes-associated islet autoantibodies and for type 1 diabetes for at least 12 months after the respective event in patients. Controls were matched by clinical site and the family history of type 1 diabetes (yes/no) if they had plasma samples at the respective time points (Table 1). Controls were randomly selected from the pool of potential controls after being matched and conditioned. Control samples used in the study were age-matched to the last islet-autoantibody-negative sample and seroconversion sample of rapid-onset patients.
Table 1.
Infection and fever reported in the infection history study
| Infection status | Patient n (%) | Control n (%) | OR (95% CI) | p value |
|---|---|---|---|---|
| Birth to type 1 diabetes | ||||
| Any infection | 24 (100) | 70 (97) | ||
| Fever | 17 (71) | 62 (86) | 0.22 (0.06, 0.88) | 0.032 |
| Fever without infectious illness | 9 (38) | 22 (31) | 1.21 (0.44, 3.30) | 0.715 |
| Fever with any infectious illness | 14 (58) | 56 (78) | 0.36 (0.12, 1.10) | 0.072 |
| Respiratory tract | 24 (100) | 68 (94) | ||
| Gastrointestinal tract | 11(46) | 29 (40) | 1.55 (0.50, 4.76) | 0.447 |
| Other | 5 (21) | 13 (18) | 1.40 (0.36, 5.48) | 0.630 |
| Autoantibody-negative period | ||||
| Any infection | 18 (75) | 53 (74) | 0.94 (0.20, 4.52) | 0.940 |
| Fever | 13 (54) | 38 (53) | 1.10 (0.31, 3.85) | 0.882 |
| Fever without infectious illness | 6 (25) | 8 (11) | 1.95 (0.59, 6.50) | 0.275 |
| Fever with any infectious illness | 9 (38) | 33 (46) | 0.76 (0.22, 2.60) | 0.658 |
| Respiratory tract | 18 (75) | 49 (68) | 1.89 (0.40, 8.89) | 0.420 |
| Gastrointestinal tract | 5 (21) | 16 (22) | 0.96 (0.27, 3.44) | 0.944 |
| Other | 2 (8) | 8 (11) | 0.57 (0.08, 3.87) | 0.564 |
| Seroconversion period | ||||
| Any infection | 17 (71) | 52 (72) | 0.95 (0.31, 2.86) | 0.922 |
| Fever | 4 (17) | 30 (42) | 0.27 (0.08, 0.88) | 0.030 |
| Fever without infectious illness | 2 (8) | 11 (15) | 0.47 (0.09, 2.56) | 0.380 |
| Fever with any infectious illness | 3 (13) | 26 (36) | 0.23 (0.06, 0.88) | 0.032 |
| Respiratory tract | 15 (63) | 49 (68) | 0.71 (0.24, 2.15) | 0.543 |
| Gastrointestinal tract | 3 (13) | 10 (14) | 1.25 (0.27, 5.84) | 0.776 |
| Other | 1 (4) | 4 (6) | 1.11 (0.10, 11.86) | 0.931 |
| Progression period | ||||
| Any infection | 17 (71) | 48 (67) | 1.34 (0.30, 6.08) | 0.706 |
| Fever | 5 (21) | 30 (42) | 0.14 (0.02, 0.85) | 0.033 |
| Fever without infectious illness | 2 (8) | 8 (11) | 0.76 (0.14, 4.13) | 0.752 |
| Fever with any infectious illness | 5 (21) | 25 (35) | 0.30 (0.07, 1.40) | 0.126 |
| Respiratory tract | 15 (63) | 43 (60) | 1.06 (0.31, 3.60) | 0.921 |
| Gastrointestinal tract | 6 (25) | 10 (14) | 2.64 (0.70, 9.95) | 0.153 |
| Other | 2 (8) | 3 (4) | 2.65 (0.33, 21.11) | 0.358 |
Infection history study
This study investigated whether infections or fever episodes were associated with rapid progression to type 1 diabetes. All 24 rapid-onset patients, with three controls for each, were examined. Controls were selected by the same procedure as for the sequencing study. Three time periods were examined in the 96 children (Fig. 1b): (1) the autoantibody-negative period, from birth to the last islet-autoantibody-negative sample; (2) The seroconversion period, from the last islet-autoantibody-negative sample to the first islet-autoantibody-positive sample; and (3) the progression period, from the first islet-autoantibody-positive sample to the date of type 1 diabetes diagnosis.
Nucleic acid sequencing
Total nucleic acids were extracted from 250 μl plasma (NucliSENS easyMag, Biomerieux, Marcy l'Etoile, France) and nucleic acid quantity and quality assessed (2100 Bioanalyzer, Agilent Technologies, Santa Clara, CA, USA). Samples were subjected to reverse transcription using random octamer primers linked to an arbitrary 17-mer primer sequence and products were randomly amplified by PCR, applying the same octamer-linked 17-mer primer in conjunction with the 17-mer primer sequence without the octamer tail in a 1:9 ratio [16]. Products of >70 bp were selected by column purification (MinElute, Qiagen, Hilden, Germany) and ligated to bar-coded linkers for sequencing on the 454 Genome Sequencer FLX (454 Life Sciences, Branford, CT, USA) without fragmentation [17]. Raw sequence reads were trimmed to remove sequences derived from amplification primers and linkers, filtered to eliminate highly repetitive sequences, and assembled into contiguous sequences, which were, together with the remaining non-assembled singleton reads, compared with the non-redundant GenBank database at the nucleotide and translated amino acid levels.
Infection history data
Acute infections or illnesses experienced between study visits were collected with the corresponding dates by parents and provided to study personnel every 3 months, starting from age 6 months. Acute infections or illnesses were recorded using ICD-10 codes (www.who.int/classifications/icd/en/) and categorised as respiratory tract, gastrointestinal or other infection. Fever was documented using one of the relevant ICD-10 codes, R50, R50.9, R50.8, or R56, and was assessed when reported with or without infection.
Statistical analysis
All infections and fever reports before type 1 diabetes onset were analysed and then assessed according to the three periods of disease progression defined in the study design (Fig. 1b). Infections and fever were either counted as present/absent in each period and child irrespective of the actual number of episodes (Table 2), or the number of episodes in each period was scored and reported as the mean per child with SD (Table 2).
Table 2.
Number of infections and fever reports per case or control in infection history study
| Patient mean (SD) | Control mean (SD) | OR (95 % CI) | p value | |
|---|---|---|---|---|
| Autoantibody-negative period | ||||
| Any infection | 3.5 (3.9) | 2.8 (3.6) | 1.22 (0.93, 1.60) | 0.150 |
| Fever | 1.0 (1.2) | 1.1 (1.5) | 0.97 (0.62, 1.53) | 0.901 |
| Seroconversion period | ||||
| Any infection | 1.7 (2.0) | 2.0 (1.9) | 0.93 (0.69, 1.25) | 0.615 |
| Fever | 0.3 (0.8) | 0.8 (1.3) | 0.61 (0.33, 1.12) | 0.108 |
| Progression period | ||||
| Any infection | 1.6 (1.5) | 1.7 (2.1) | 0.95 (0.66, 1.37) | 0.782 |
| Fever | 0.4 (0.9) | 0.7 (0.9) | 0.57 (0.26, 1.21) | 0.143 |
Conditional logistic regression adjusted for maternal age at delivery was used to obtain the estimate of OR for the factor of interest. A p value <0.05 was considered significant. All reported p values are two-sided without adjustment for multiple testing. All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Sequencing study
Unbiased sequencing identified viruses in six (11%) of 56 plasma samples (electronic supplementary material [ESM] Table 1). Samples from four (14%) of the 28 children analysed (one patient, three controls) were positive for virus. The positive patient sample contained a picornavirus, human rhinovirus C (HRV-C), identified in the last islet-autoantibody-negative sample. Rhinoviruses are commonly associated with upper respiratory tract infection and otitis media, and not known to occur in blood. However, the species rhinovirus C differs from species rhinovirus A and B [18], and has been reported in blood with a peak of viremia two days after the onset of symptoms [19]. Fever was not reported for this patient during the autoantibody-negative period, but three respiratory infections were reported, with the last one recorded 1 day before sampling. Three different viruses were detected in the autoantibody-negative controls. Human parvovirus B19 (B19V), the causative agent of fifth disease (erythema infectiosum, ‘slapped cheek syndrome’ [20]) was identified in the paired control of the virus-positive case, with fever reported 30 days before sampling; no rash was recorded for this child. In the second positive control child, both samples tested contained human herpesvirus 6 (HHV-6), which can establish latent infection. Two species of HHV-6 are recognised: HHV-6B is considered the causative agent of sixth disease (exanthema subitum or roseola infantum [21]), while little is known about the pathogenicity of the less frequently diagnosed HHV-6A. The sequence identified in this child was distantly related to HHV-6A. The third control child was positive in both plasma samples for GB virus C (GBV-C), which is known to establish persistent infections consistent with detection over a 3 month time period [22].
Infection history study
All rapid-onset patients and 70 of 72 controls reported at least one infection prior to the onset of type 1 diabetes (Table 1). The proportions of patients and controls with infections reported during the islet-autoantibody-negative (75% vs 74%), seroconversion (71% vs 72%) and progression periods (71% vs 67%) were similar. Moreover, the number of infections per child during each of these periods was similar between patients and controls (Table 2). Respiratory infections were the most commonly reported infections in both patients and controls.
In contrast to the reported infections, the frequency of reported fever episodes differed between patients and controls (Table 1). Overall, fever was reported less frequently in patients than in controls. Although the difference was seen during the seroconversion (p=0.030) and progression periods (p=0.033), it was not present during the autoantibody-negative period.
Discussion
Infection during infancy is a candidate trigger of islet autoimmunity and type 1 diabetes [3-5]. In TEDDY participants who progressed rapidly to diabetes, unbiased sequencing capable of detecting any possible agent failed to detect viraemia around the time of seroconversion more frequently than in controls. Rapid progression to diabetes was also not associated with reported infectious episodes. We had hypothesised that rapidly progressing patients would provide a best-case scenario for detecting potentially associated viruses in blood, because of the fulminant presentation of diabetes. However, viraemia was detected in only one of 14 patients. These findings cannot exclude the possibility that a causative virus is acquired before the age of 6 months and absent or only present for a brief time in plasma during the months leading to the appearance of islet autoantibodies and progression to diabetes. Although the sensitivity of the sequencing approach may be limited in detecting a low-grade persistent infection, next-generation sequencing techniques have been successful in identifying viral causes of disease in several scenarios [23-26]. In TEDDY, consistent detection of the same virus was seen for two consecutive samples from two children, suggesting that this method can reproducibly detect viraemia in plasma samples. Retrospective quantification of identified viruses in the analysed plasma samples by specific real-time PCR indicated target loads down to a range of 200-1000 molecules/250 μl plasma, a detection limit suitable to detect active systemic infection.
A limitation of our study was the relatively low number of cases studied and the length of the sampling interval which, at 3 months, may have been too wide to identify all systemic infections. Viraemia can be as short as a few days, and additional samples collected during fever episodes would have been desirable. However, ad hoc sampling aside from the scheduled TEDDY visits was not part of the TEDDY study protocol. Although infections were reported frequently in our children, the analysis of infection history data did not support a prominent role of infection; infection frequencies were similar in patients and controls during all three observation periods and for all three infection categories.
The lower frequency of fever reported for patients than for controls is a potentially important finding that needs to be substantiated in a larger study. If confirmed, it suggests a protective effect of fever as a marker of more vigorous infection defence and effective virus elimination.
In conclusion, next-generation sequencing of the plasma virome of patients rapidly developing autoimmune type 1 diabetes in early childhood did not provide evidence for viraemia around the time of seroconversion in these children.
Supplementary Material
Acknowledgements
The authors thank: V. Kapoor, A. Petrosov, A. Shah and A. Tashmukhamedova (Center for Infection and Immunity, Mailman School of Public Health, Columbia University, New York, NY, USA) for expert technical assistance; J. E. Herrera and K. Jain (Center for Infection and Immunity, Mailman School of Public Health, Columbia University, New York, NY, USA) for bioinformatics support; and M. Bhat for project management at CII (Center for Infection and Immunity, Mailman School of Public Health, Columbia University, New York, NY, USA).
Funding
The study was funded by DK 63829, 63861, 63821, 63865, 63863, 63836, 63790 and UC4DK095300 and contract number HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and Centers for Disease Control and Prevention (CDC).
Abbreviations
- ICD-10
International Classification of Disease (ICD)-10
- TEDDY
The Environmental Determinants of Diabetes in the Young
Footnotes
Contribution statement
AGZ, EB JK, OS, JXS, WH and BA designed this project within the TEDDY study. HSL, AGZ and EB performed statistical analyses. TB performed sequencing and viral analyses. CW, MP and HSL acquired and reviewed data. HH and MR were involved in the interpretation of results. AGZ, MR, OS, JXS, WH and ÅL are principal investigators of the TEDDY study and acquired samples and reviewed the data. AGZ, HL, EB, TB, CW and MP wrote the manuscript. All authors critically reviewed the manuscript for intellectual content and approved the final manuscript
Duality of interest
The authors confirm that there is no duality of interest associated with this manuscript.
References
- 1.Ziegler AG, Nepom GT. Prediction and pathogenesis in type 1 diabetes. Immunity. 2010;32:468–478. doi: 10.1016/j.immuni.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 3.Roivainen M, Klingel K. Virus infections and type 1 diabetes risk. Curr Diab Rep. 2010;10:350–356. doi: 10.1007/s11892-010-0139-x. [DOI] [PubMed] [Google Scholar]
- 4.Tauriainen S, Oikarinen S, Oikarinen M, Hyoty H. Enteroviruses in the pathogenesis of type 1 diabetes. Semin Immunopathol. 2011;33:45–55. doi: 10.1007/s00281-010-0207-y. [DOI] [PubMed] [Google Scholar]
- 5.Stene LC, Rewers M. Immunology in the clinic review series; focus on type 1 diabetes and viruses: the enterovirus link to type 1 diabetes: critical review of human studies. Clin Exp Immunol. 2012;168:12–23. doi: 10.1111/j.1365-2249.2011.04555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 7.Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci USA. 2007;104:5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stene LC, Oikarinen S, Hyöty H, et al. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY). Diabetes. 2010;59:3174–3180. doi: 10.2337/db10-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikarinen S, Martiskainen M, Tauriainen S. Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes. 2011;60:276–279. doi: 10.2337/db10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Füchtenbusch M, Irnstetter A, Jäger G, Ziegler AG. No evidence for an association of coxsackie virus infections during pregnancy and early childhood with development of islet autoantibodies in offspring of mothers or fathers with type 1 diabetes. J Autoimmun. 2001;17:333–340. doi: 10.1006/jaut.2001.0550. [DOI] [PubMed] [Google Scholar]
- 11.Mercalli A, Lampasona V, Klingel K, et al. No evidence of enteroviruses in the intestine of patients with type 1 diabetes. Diabetologia. 2012;55:2479–2488. doi: 10.1007/s00125-012-2591-4. [DOI] [PubMed] [Google Scholar]
- 12.Simonen-Tikka ML, Pflueger M, Klemola P, et al. Human enterovirus infections in children at increased risk for type 1 diabetes: the Babydiet study. Diabetologia. 2011;54:2995–3002. doi: 10.1007/s00125-011-2305-3. [DOI] [PubMed] [Google Scholar]
- 13.The TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8:286–298. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 14.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95:3360–3367. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puavilai G, Chanprasertyotin S, Sriphrapradaeng A. Diagnostic criteria for diabetes mellitus and other categories of glucose intolerance: 1997 criteria by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (ADA), 1998 WHO consultation criteria, and 1985 WHO criteria. World Health Organization. Diabetes Res Clin Pract. 1999;44:21–26. doi: 10.1016/s0168-8227(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 16.Quan PL, Palacios G, Jabado OJ, et al. Detection of respiratory viruses and subtype identification of influenza A viruses by GreeneChipResp oligonucleotide microarray. J Clin Microbiol. 2007;45:2359–2364. doi: 10.1128/JCM.00737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margulies M, Egholm M, Altman WE, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renwick N, Schweiger B, Kapoor V, et al. A recently identified rhinovirus genotype is associated with severe respiratory tract infection in children in Germany. J Infect Dis. 2007;196:1754–1760. doi: 10.1086/524312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuji N, Suzuki A, Lupisan S, et al. Detection of human rhinovirus C viral genome in blood among children with severe respiratory infections in the Philippines. PLoS ONE. 2011;6(11):e27247. doi: 10.1371/journal.pone.0027247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350:586–597. doi: 10.1056/NEJMra030840. [DOI] [PubMed] [Google Scholar]
- 21.Yamanishi K, Okuno T, Shiraki K, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 22.Feucht HH, Zöllner B, Polywka S, et al. Distribution of hepatitis G viremia and antibody response to recombinant proteins with special regard to risk factors in 709 patients. Hepatology. 1997;26:491–494. doi: 10.1002/hep.510260234. [DOI] [PubMed] [Google Scholar]
- 23.Palacios G, Druce J, Du L, et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N Engl J Med. 2008;358:991–998. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- 24.Briese T, Paweska JT, McMullan LK. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 2009;5:e1000455. doi: 10.1371/journal.ppat.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang P, Chiu C. Metagenomics for the discovery of novel human viruses. Future Microbiol. 2010;5:177–189. doi: 10.2217/fmb.09.120. [DOI] [PubMed] [Google Scholar]
- 26.Xu B, Liu L, Huang X, et al. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province, China: discovery of a new bunyavirus. PLoS Pathog. 2011;7:e1002369. doi: 10.1371/journal.ppat.1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.