Table 1.
Evaluation of reaction parameters.
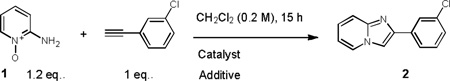 | |||
|---|---|---|---|
| Entry | Catalyst[a] | Additive | Conversion[b] |
| 1 | PicAuCl2 | None | 43 |
| 2 | PicAuCl2 | MsOH (1 eq.) | 71 |
| 3 | PicAuCl2 | TFA (1 eq.) | 70 |
| 4 | None | TFA (1 eq.) | none |
| 5 | PicAuCl2 | p-NO2PhCO2H (1 eq.) | none |
| 6 | PicAuCl2 | HNTf2 (1 eq.) | 72 |
| 7 | PicAuCl2 | TFA (0.5 eq.) | 54 |
| 8 | AuCl | TFA (1 eq.) | 33 |
| 9 | AuCl3 | TFA (1 eq.) | 53 |
| 10 | NHCAuNTf2 | TFA (1 eq.) | 36 |
| 11 | PPh3AuNTf2 | TFA (1 eq.) | 36 |
| 12 | PicAuCl2 | TFA (1 eq.)[c] | 89 (72)[d] |
10 mol % of catalyst was used.
Determined by correlation between HPLC-MS and crude 1H-NMR analysis using 3,5-dinitrobenzoic acid as internal standard.
The reaction mixture was heated at 40°C for 15h.
Isolated yield after column chromatography.
