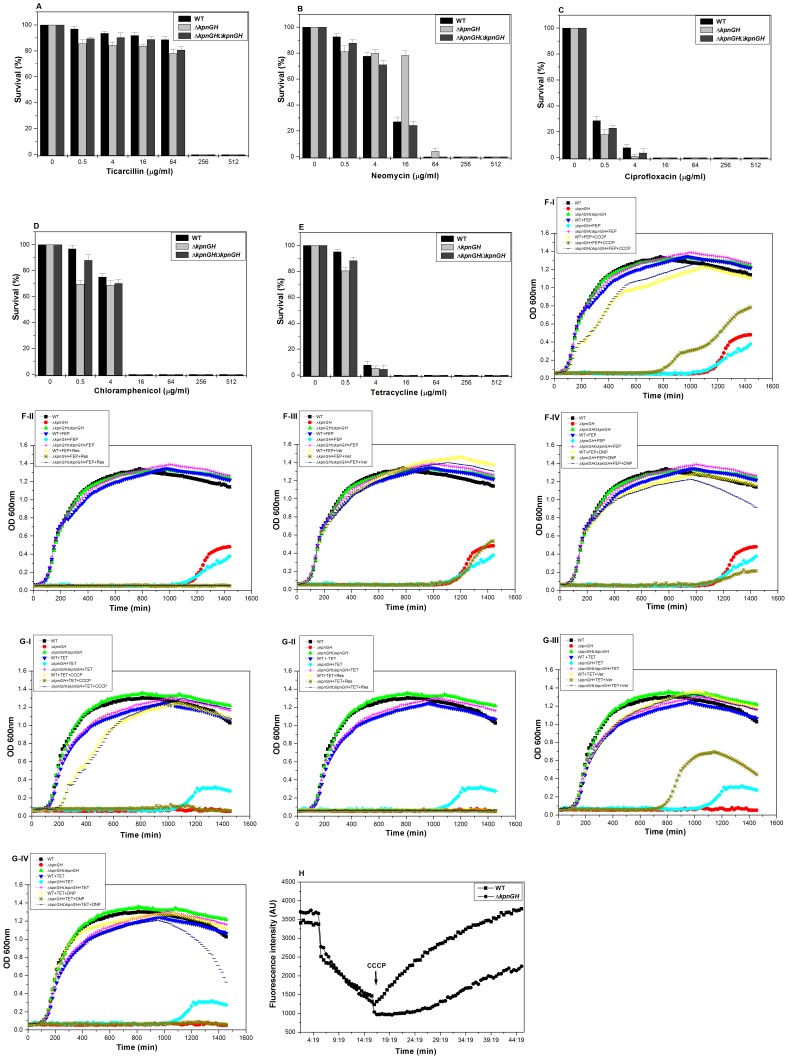
Figure 4. Susceptibility, Growth inactivation and accumulation assays towards different antibiotics.
A) Sensitivity of WT, ΔkpnGH and ΔkpnGHΩkpnGH towards ticarcillin. Upon exposing the cells to different concentrations of ticarcillin it was observed that the total CFU count of WT at 0.5 µg/ml was 1 fold, 4 µg/ml was 1.13 fold, 16 µg/ml was 1.11 fold, 64 µg/ml was 1.09 fold, 256 µg/ml was 1.09 fold, 512 µg/ml was 1.13 fold increased than ΔkpnGH [P = 0.027]. The data is the means of measurements made in triplicate and performed three times. B) Sensitivity of WT, ΔkpnGH and ΔkpnGHΩkpnGH towards neomycin. Upon exposing the cells to different concentrations of neomycin it was observed that the total CFU count of WT at 0.5 µg/ml was 1.14 fold, 4 µg/ml was 0.97 fold, 16 µg/ml was 0.34 fold increased than ΔkpnGH [P = 0.042]. The data is the means of measurements made in triplicate and performed three times. C) Sensitivity of WT, ΔkpnGH and ΔkpnGHΩkpnGH towards ciprofloxacin. Exposing the cells to different concentrations of ciprofloxacin it was observed that the total CFU count of WT at 0.5 µg/ml was 1.5 fold, 4 µg/ml was 7.3 fold increased than ΔkpnGH [P = 0.0188]. The data is the means of measurements made in triplicate and performed three times. D) Sensitivity of WT, ΔkpnGH and ΔkpnGHΩkpnGH towards chloramphenicol. Exposing the cells to different concentrations of chloramphenicol it was observed that the total CFU count of WT at 0.5 µg/ml was 1.39 fold, 4 µg/ml was 1.08 fold increased than ΔkpnGH [P = 0.0266]. The data is the means of measurements made in triplicate and performed three times. E) Sensitivity of WT, ΔkpnGH and ΔkpnGHΩkpnGH towards tetracycline. Exposing the cells to different concentrations of tetracycline it was observed that the total CFU count of WT at 0.5 µg/ml was 1.18 fold, 4 µg/ml was 1.45 fold increased than ΔkpnGH [P = 0.0288]. The data is the means of measurements made in triplicate and performed three times. F) Growth inactivation assay using wild type, ΔkpnGH and ΔkpnGHΩkpnGH in the presence of cefepime. Growth pattern in absence of any drug or inhibitor is included as control. The growth rate of ΔkpnGH in the presence of 0.1 µg/ml cefepime, was 15.373 fold lesser when compared to that of WT (P = 0.000574). A decrease in growth was observed in kpnGH mutant (7.474-fold; P = 0.000123), (1.032-fold; P = 0.000159), (16.23-fold; P = 0.000215) and (12.5-fold; P = 0.000101) in the presence of CCCP (I), reserpine (II), verampamil (III) and 2, 4 DNP (IV) respectively. The mean values of three independent experiments have been used for plotting the graph. G) Growth inactivation assay using wild type, ΔkpnGH and ΔkpnGHΩkpnGH in the presence of tetracycline. Growth pattern in absence of any drug or inhibitor is included as control. The growth rate of ΔkpnGH in the presence of 0.01 µg/ml tetracycline, was 10.655 fold lesser when compared to that of WT (P = 0.000234). A decrease in growth was observed in kpnGH mutant (10.476-fold; P = 0.000477), (1.102-fold; P = 0.000796), (8.134-fold; P = 0.000101) (15.28-fold; P = 0.000101) in the presence of CCCP (I), reserpine (II), verampamil (III) and 2, 4 DNP (IV) respectively. The mean values of three independent experiments have been used for plotting the graph. H) Accumulation studies using EtBr with control strain and ΔkpnGH. Efflux of EtBr in mutant and wild type cells was monitored continuously by measuring fluorescence emission at 600 nm upon excitation at 530 nm. After 5 mins in flourimeter, cells loaded with EtBr were energized by addition of glucose and efflux of EtBr was monitored. After 10 mins, 100 µM CCCP was added as indicated to abolish active transport and fluorescence emission was monitored further. The fluorescence was measured using spectrofluorometer (Hitachi). Each data point represents the mean plus the standard deviation of three independent experiments.