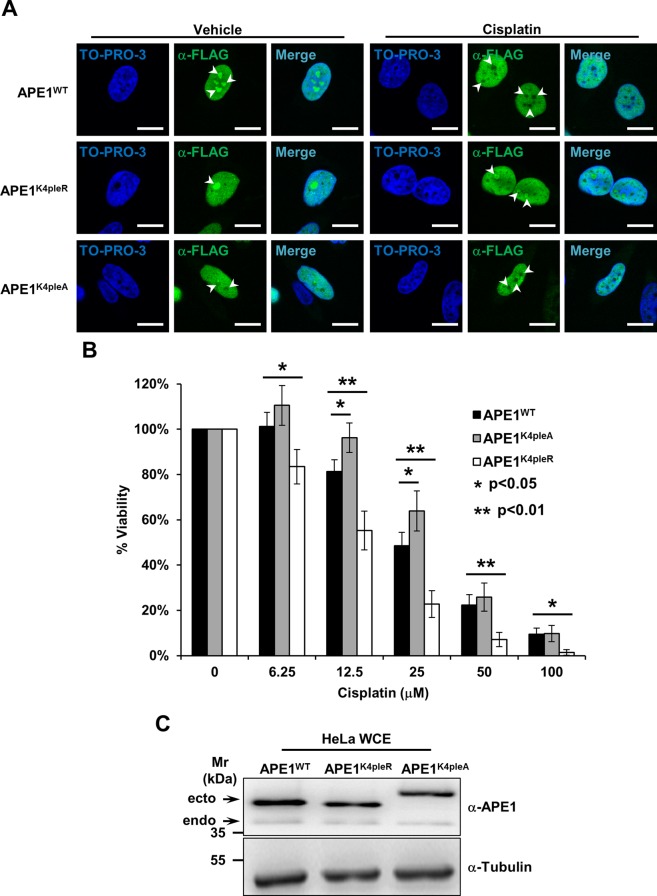
FIGURE 7:
APE1 redistribution upon cisplatin requires active modification of lysine residues and protects cells from cisplatin cytotoxicity. (A) Representative immunofluorescence analysis on HeLa cells expressing different FLAG-tagged APE1 forms. On cisplatin treatment (100 μM for 6 h) only APE1WT redistributes from nucleoli (white arrowheads), whereas the nonacetylatable APE1K4pleR does not display major relocalization. The APE1K4pleA form fails to accumulate within nucleoli, regardless of the cisplatin treatment. TO-PRO-3 counterstaining was used to highlight nuclei; bars, 16 μm. (B) Viability assay on HeLa cell clones expressing the indicated APE1 forms. Cells were treated with doxycycline to induce endogenous APE1 knockdown (Lirussi et al., 2012) and challenged with increasing amounts of cisplatin for 24 h. The histogram reports the mean ± SD of at least three independent experimental replicates. The APE1K4pleR-expressing clone is hypersensitive to cisplatin, indicating that modification on critical APE1 lysine residues and redistribution of the protein from nucleoli are important to cell survival. The APE1K4pleA-expressing cells, conversely, show a protective phenotype linked to a decreased cell proliferation rate. (C) Representative Western blot analysis on HeLa cell clones used for the experiments in B. HeLa whole-cell extracts (20 μg) from the indicated clones were probed with an anti-APE1 antibody. The analysis shows comparable expression of the ectopic APE1 forms (ecto) and minimum residual of the endogenous protein (endo). Tubulin was used as loading control.