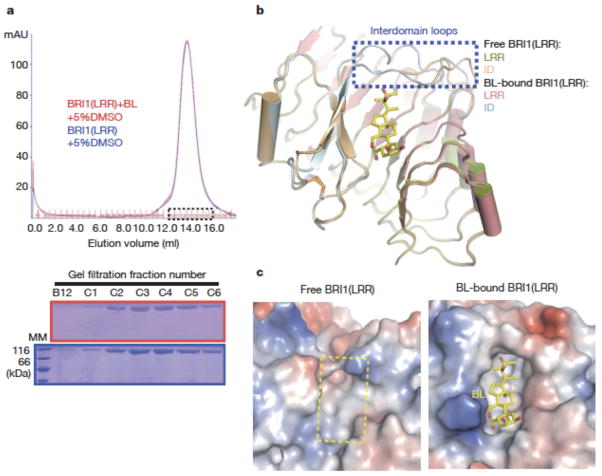
Fig. 4. BL induces stabilization of two inter-domain loops but no dimerization of BRI1-LRR.
a. BL has no effect on the oligomeric status of BRI1-LRR in solution. Shown on the top is superposition of the gel filtration chromatograms of BRI1-LRR in the absence (blue) and presence (red) of BL. The vertical and horizontal axes represent UV absorbance (λ=280 nm) and elution volume respectively. Peak fractions are highlighted within the dashed square. The apparent molecular weight of BRI1-LRR was 109.4 kD in the presence or absence of BL, higher than the theoretical BRI1-LRR monomer (83 kD) likely due to existence of multiple glycosylation sites in BRI1-LRR. MM: molecular mass marker. Bottom: Coomassie blue staining of the peak fractions shown on the top following SDS-PAGE.
b. BL binding induces stabilization of two inter-domain loops. Structural superimposition of the free and BL-bound BRI1-LRR around the BL-binding site.
c. BL binding generates a striking hydrophobic surface groove on BRI1-LRR. Shown on the left and right are the electrostatic surfaces of free BRI1-LRR and BL-bound BRI1-LRR (shown in the same orientation) around the BL-binding site respectively. The area highlighted with the yellow dashed square on the left panel is the BL-binding site. White, blue and red indicate neutral, positive and negative surfaces respectively.