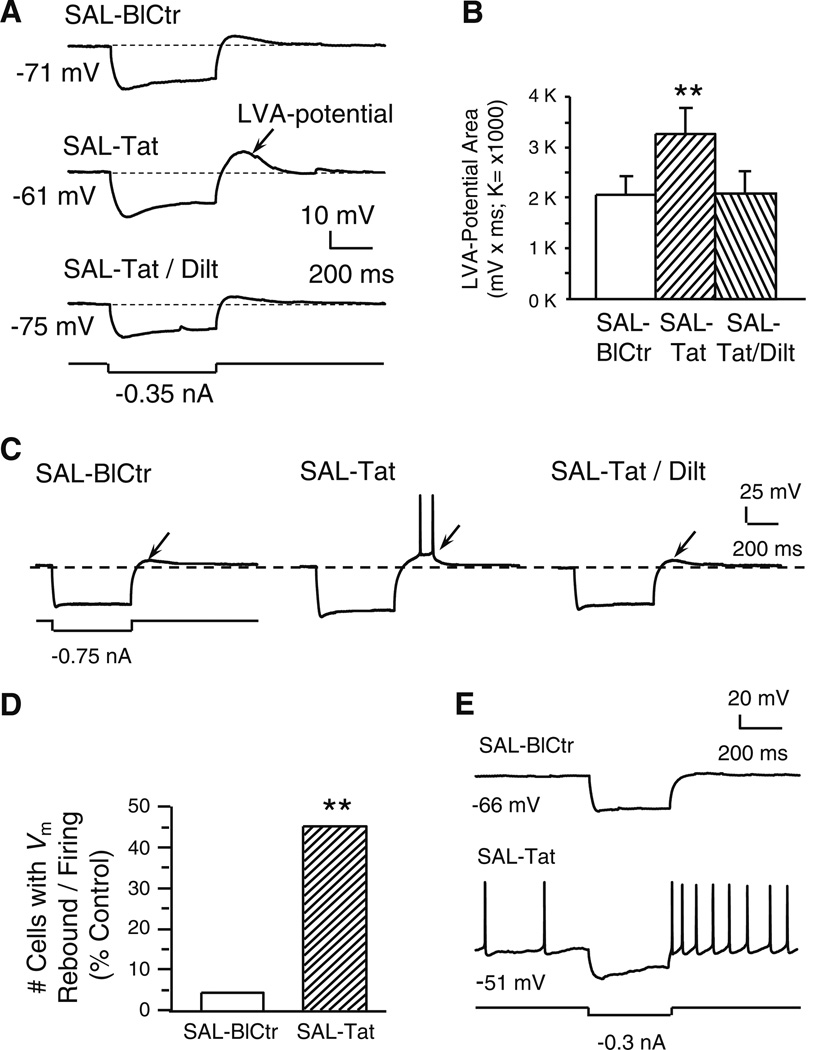
Fig. 3. Tat potentiated a subthreshold, low voltage-activated (LVA-) membrane potential and triggered spiking; both changes were abolished by diltiazem (indicated by arrows).
All recordings were in slices obtained from SAL-treated rats. a. Recording traces illustrate that a marked membrane hyperpolarization (more negative than −110 mV) was immediately followed by a small, Vm rebound (i.e., a subthreshold, low voltage-activated (LVA)-potential; upper trace). Tat (40 nM) markedly enlarged this LVA potential (middle trace). The Tat-induced effect was abolished by concurrent bath application of diltiazem (40 nM; lower trace). b. Quantification of LVA-potential area. Tat (40 nM) significantly enlarged the subthreshold LVA-potential area and this effect was abolished by co-perfusion of diltiazem (40 nM) (n=9 neurons recorded from 9 rats; one-way rmANOVA, p=0.008; post hoc Dunnett’s test, **p<0.01). c. Traces from a single neuron illustrating that Tat-induced LVA-potential enhancement achieved the firing threshold and elicited spontaneous action potentials. d. Number of neurons that displayed the LVA-potential and spontaneous firing triggered by the LVA-Vm rebound; data are presented as%of total neurons tested (n=22 from 17 rats). Only one neuron showed the Vm rebound-triggered firing prior to Tat perfusion. During Tat (40 nM) application, 10/22 neurons demonstrated Vm rebound-triggered firing (Chi-squared test, **p<0.01). e. In two of the ten neurons showing the Vm rebound (illustrated are traces from one of these two), Tat (40 nM) depolarized the RMP to spiking threshold so that spontaneous firing occurred, and spiking was further enhanced by the Vm rebound in response to a hyperpolarizing stimulus