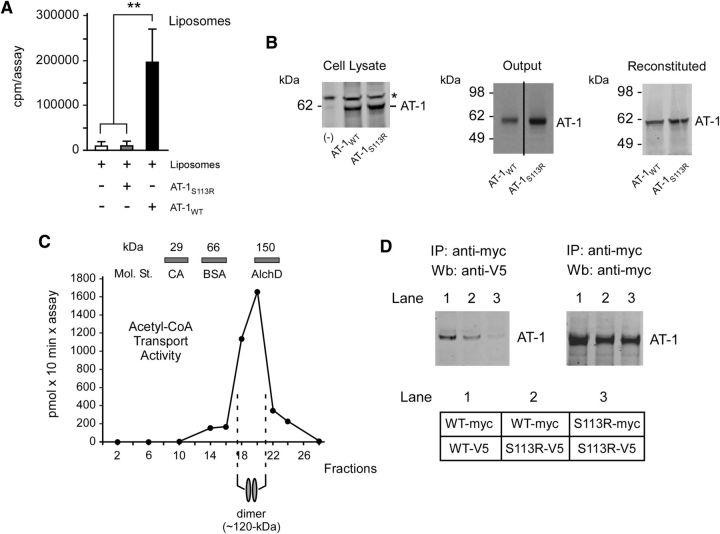
Figure 1.
AT-1S113R is unable to form homodimers in the membrane and is deficient of acetyl-CoA transport activity. A, Functional reconstitution of affinity-purified AT-1 into artificial liposomes. AT-1S113R is devoid of transport activity. Values are the mean (n = 3) ± SD. B, Immunoblot showing levels of AT-1WT and AT-1S113R in cell lysates, after affinity purification (output), and after reconstitution. Lanes in the output are from the same membrane. Asterisk (*) indicates a background band visible in both transfected and nontransfected (-) cells. C, Endogenous AT-1 migrates as a homodimer on analytical ultracentrifugation. Values are the mean (n = 4) ± SD. Bars on top show the sedimentation of molecular standards (Mol. St.): carbonic anhydrase (CA; 29 kDa), BSA (66 kDa) and alcohol dehydrogenase (AlchD; 150 kDa). D, Immunoblots showing co-IP of myc- and V5-tagged versions of AT-1WT and AT-1S113R. AT-1WT forms homodimers while AT-1S113R does not. A schematic view of the experiment is shown in the bottom.