Figure 2.
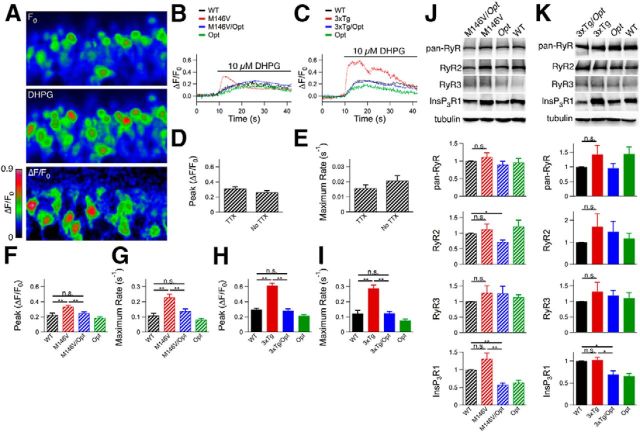
Ex vivo Oregon Green Ca2+ imaging in hippocampal dentate gyrus. A, Hippocampal slices were obtained from 10- to 12-d-old animals, loaded with Oregon Green, and dentate gyrus granular cell layer neurons were imaged by confocal microscopy. After obtaining a baseline recording (F0), the solution was switched to aCSF containing 10 μm dihydroxyphenylglycine (DHPG) for the remainder of the recording period. B, C, Representative traces for M146V lines (B) and 3xTg lines (C). D, E, Magnitudes (D) and rates (E) of Oregon Green fluorescence change in dentate gyrus granular cell layer neurons in WT mice (C57BL/6 background) with and without tetrodotoxin present. Unpaired two-tailed t test, n = 102–125 responding neurons from two mice, no significant differences observed. F, H, Magnitudes of Oregon Green fluorescence change after DHPG perfusion for M146V lines (F) and 3xTg lines (H). Unpaired two-tailed t test, n = 72–207 responding neurons from ≥ 3 mice each, p < 0.005. G, I, Rates of Oregon Green fluorescence change after DHPG perfusion for M146V lines (G) and 3xTg lines (I). Unpaired two-tailed t test, n = 72–207 responding neurons from ≥ 3 mice each, p < 0.005. J, K, Western blot analyses of hippocampal lysates from P10–P12 mice from M146V (n = 6 mice each; J) and 3xTg (n ≥ 6 mice each; K) lines using a pan-RyR antibody or antibodies specific to RyR2, RyR3, or InsP3R1, and tubulin as a loading control. Unpaired two-tailed t test, *p < 0.05, **p < 0.005. Error bars show mean and SEM.