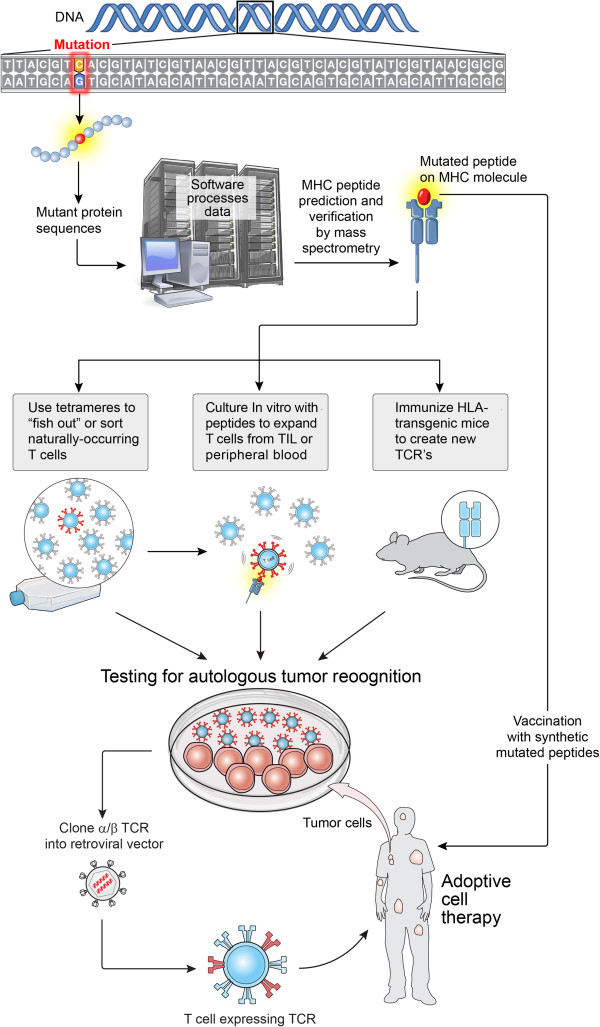
Figure 1.
Highly personalized medicine. Inexpensive and highly available DNA sequencing can revolutionize cancer immunotherapy by enabling highly personalized approaches involving the identification of new tumor-associated antigens. The expressed genes from a patient’s tumor can be sequenced to identify candidate mutant T cell epitopes. Relevant epitopes that could potentially bind to any given patient’s HLA molecules could be predicted using peptide prediction algorithms (e.g. http://www.syfpeithi.de/bin/MHCServer.dll/EpitopePrediction.htm. Or http://www-bimas.cit.nih.gov/molbio/hla_bind). If peptides derived from mutant proteins are found to capable of forming new HLA-restricted target structures, the candidate peptides can be used in one of at least several ways: 1) “fish out” or sort cells for relevant antigens (such as those specific for driver oncogenes) using tetramer like reagents; 2) use the candidate peptides to stimulate T cell clonotypes already present in a patient’s tumor or in their peripheral blood; 3) use antigens to elicit new T cell receptors in mice that are transgenic for human MHC molecules; and 4) to immunize patients against antigens. If the T cells generated are specific for a patient’s tumor, they can be expanded and adoptively transferred if they are of human origin, or used as a source of TCR for gene engineering approaches.