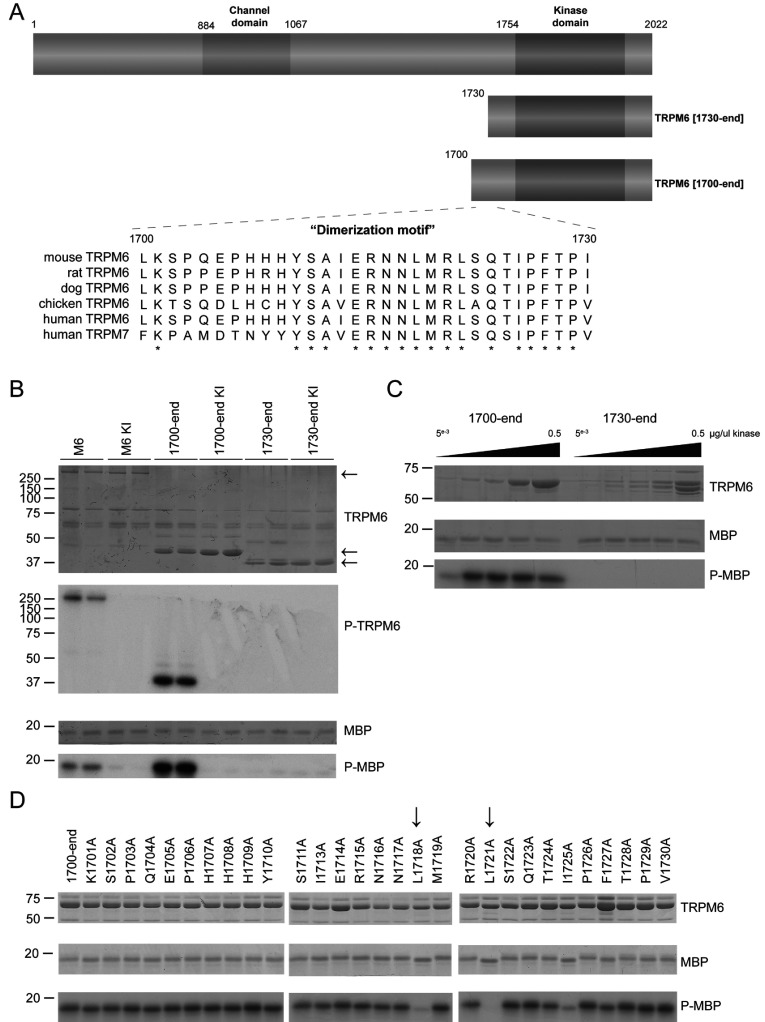
Figure 1. Characterization of TRPM6 (auto)phosphorylation in C-terminal truncation mutants.
(A) Schematic representation of the domain structure of TRPM6 demonstrating the ion channel domain and the α-kinase domain. The lower panel demonstrates a multiple sequence alignment of the 1700–1730 region of TRPM6 among different species along with human TRPM7. Asterisks represent conserved amino acids. (B) The Coomassie Blue-stained SDS gel indicates the FLAG-tagged full-length and truncated forms of wild-type and kinase-inactive (KI) TRPM6. Phosphorylation of MBP and TRPM6 itself was determined by autoradiography (lower panels). (C) Increasing amounts of GST-purified kinase fragments TRPM6-(1700–end) and TRPM6-(1730–end) were subjected to a kinase assay using MBP as substrate. Proteins were separated by SDS/PAGE, stained with Coomassie Blue (top and middle panels) and phosphorylation was detected by autoradiography (bottom panel). (D) Alanine-scanning mutagenesis has been performed on the 1700–1730 region. The indicated GST-tagged mutants were immunoprecipitated from overexpressed HEK-293 cells, and kinase activity was assayed using MBP as substrate. Proteins were separated by SDS/PAGE, stained with Coomassie Blue (top and middle panels) and phosphorylation was detected by autoradiography (bottom panel). Molecular masses are indicated in kDa.