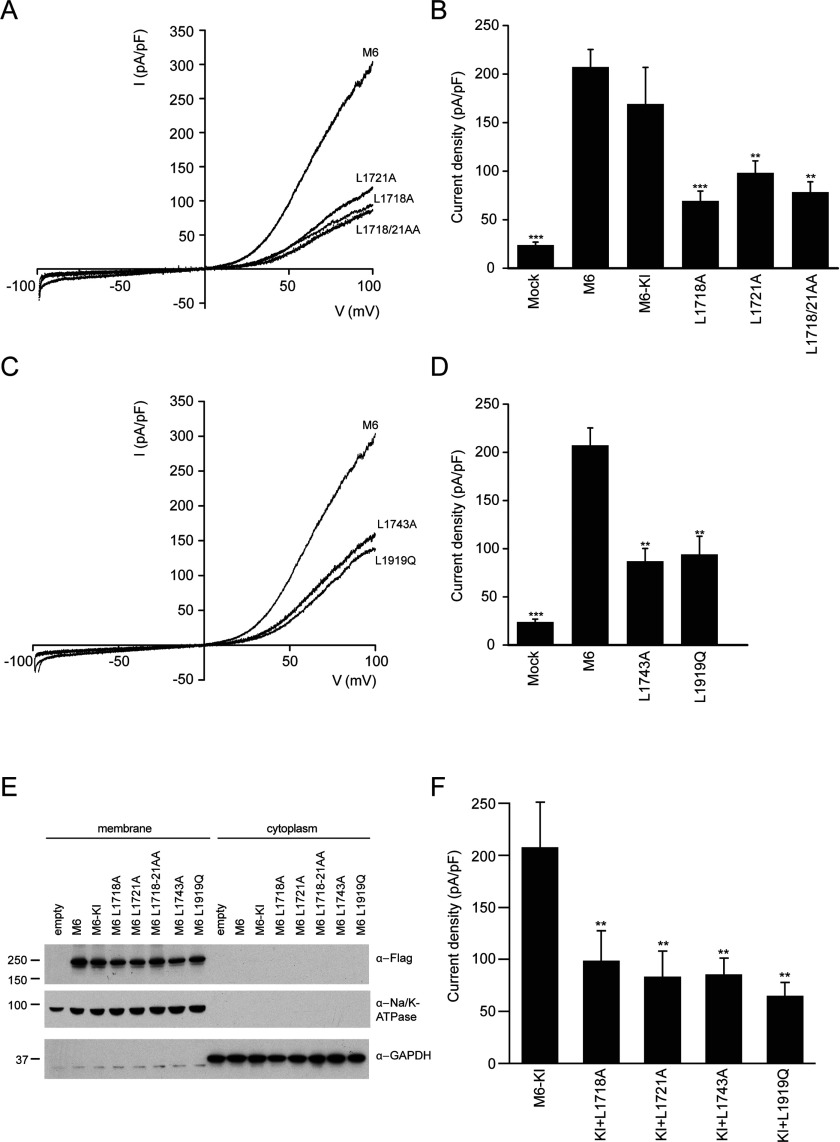
Figure 4. Dimerization interactions are linked to the channel function.
(A) Representative current recorded after 200 s of stimulation by a voltage ramp between −100 and +100 mV of HEK-293 cells transfected with wild-type and kinase-inactive (KI) TRPM6 and the indicated mutants (L1718A, L1721A and L1718/21AA). (B) Histogram summarizing the current density (pA/pF) at +80 mV of TRPM6 and indicated mutants. **P<0.05 and ***P<0.01 compared with wild-type TRPM6 (n=11–50 cells). (C) Representative current recorded after 200 s of stimulation by a voltage ramp between −100 and +100 mV of HEK-293 cells transfected with TRPM6 and the indicated mutants (L1743A and L1919Q). (D) Histogram summarizing the current density (pA/pF) at +80 mV of TRPM6 and the indicated mutants. **P<0.05 and ***P<0.01 compared with wild-type TRPM6 (n=11–50 cells). (E) Membrane and cytoplasmic fractions were obtained by ultracentrifugation to determine the expression of FLAG-tagged TRPM6 and all mutants. Lysates were immunoblotted with anti-FLAG antibody, and Na+/K+-ATPase and GAPDHs used as membrane and cytosolic marker were respectively. Molecular masses are indicated in kDa. (F) Histogram summarizing the current density (pA/pF) at +80 mV of kinase-inactive (KI) TRPM6 and indicated double mutants (KI+L1718A, KI+L1721A, KI+L1743A and KI+L1919Q). **P<0.05 compared with TRPM6 KI (n=13–25 cells). Results are means ± S.E.M.