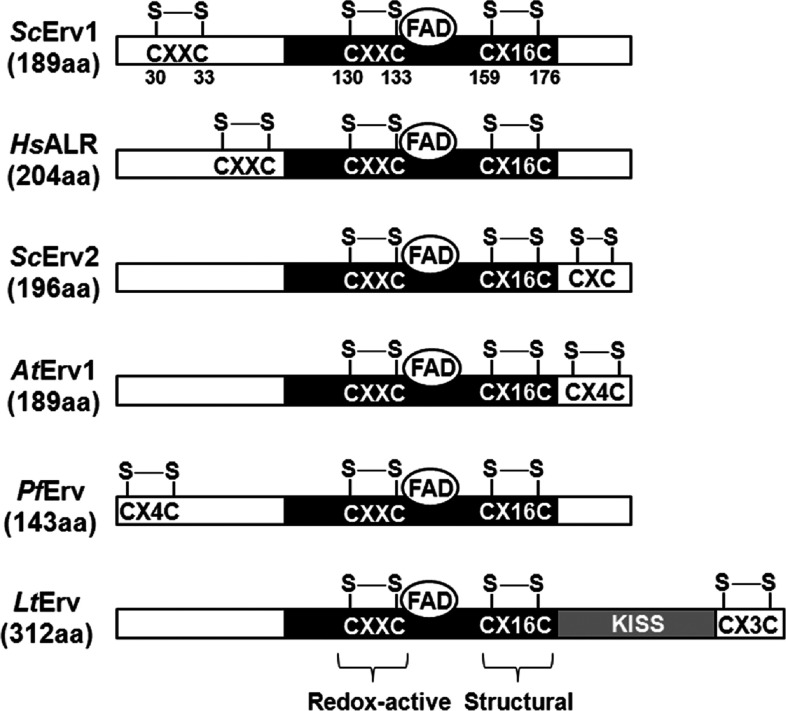
Figure 1. Schematic sequence alignment of ERV/ALR homologues.
The conserved FAD-binding catalytic domain (black) contains a redox-active CXXC disulfide and a structural CX16C disulfide. An additional shuttle disulfide is located at non-conserved domain (white). The architecture of LtErv is slightly altered with a KISS (Kinetoplastida-specific second) domain (grey) of approximately ~200 amino acids at the C-terminus. Hs, Homo sapiens; Sc, S. cerevisiae.