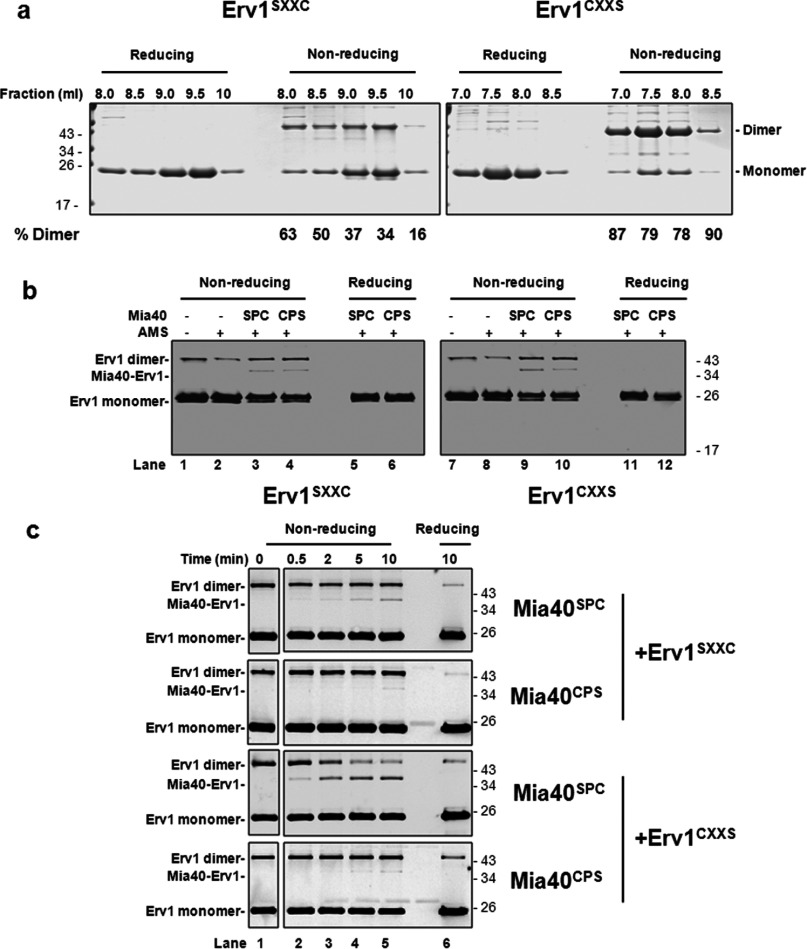
Figure 6. Cys30 is more reactive than Cys33 for intermolecular disulfide bond formation.
(a) Tris/Tricine SDS/PAGE (16% gel) of Erv1SXXC and Erv1CXXS mutant protein peak fractions in Figure 5(a) under reducing and non-reducing conditions. (b) Mixed disulfide bond formation between single cysteine mutants of Mia40 CPC (Mia40SPC and Mia40CPS) and Erv1 shuttle cysteine residues (Erv1SXXC and Erv1CXXS). The mutant proteins were pre-treated briefly with 2 mM TCEP and buffer-exchanged to buffer A before incubation at equimolar concentrations of 5 μM for 20 min at room temperature. The reactions were stopped by the addition of sample buffer with 1 mM DTT or 2.5 mM AMS. The proteins were detected by Western blotting with an antibody against Erv1. (c) Time courses of mixed disulfide bond formation between Mia40 and the Erv1 mutants. The proteins were treated as in (b) over a 10-min incubation. The molar ratio concentration of Mia40 to Erv1 used was 10:1 (50 μM Mia40 and 5 μM Erv1). The reactions were stopped at each time point by addition of sample buffer containing 20 mM IAM. As a reducing control, reactions at the end of each time course were analysed under reducing conditions by resuspending the reaction mixtures in sample buffer containing DTT.