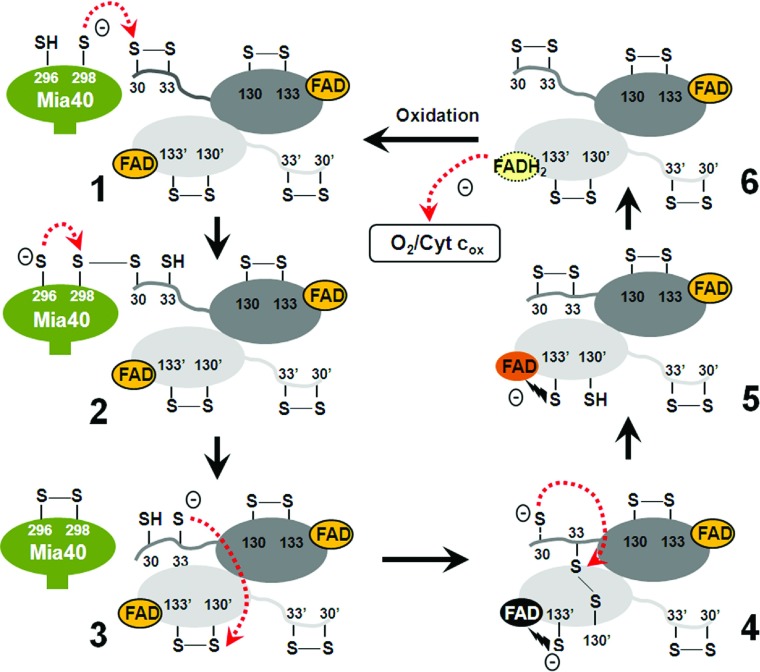
Figure 9. Model of thiol–disulfide exchange reactions in Erv1 catalysis.
This model is focused on the reductive half-reaction. Thiolate anion (S−) of the second cysteine (Cys298) of the Mia40 CPC motif nucleophilically attacks Cys30 of Erv1 (stage 1), leading to the formation of Mia40–Erv1 mixed disulfide bond, which is then attacked by S− of the first cysteine (Cys296) of Mia40 for oxidation of the CPC motif and reduction of the Erv1 Cys30–Cys33 disulfide bond (stage 2). Thiolate of Cys33 nucleophilically attacks Cys130′ of the other subunit of Erv1 (stage 3), leading to the formation of an intermediate Cys33–Cys130′ disulfide bond between two Erv1 subunits and a thiolate to FAD CTC (stage 4). Then, the Cys33–Cys133′ disulfide is reduced via nucleophilic attack on Cys33 by Cys30 (step 4), which leads to reoxidation of the Cys30–Cys33 disulfide bond (stage 5). Subsequently, a pair of electrons from the reduced redox active-site Cys130′/Cys133′ is transferred to FAD coupled with the reformation of the Cys130′–Cys133′ disulfide bond (stage 6). Finally, the oxidative half-reaction for Erv1 turnover is completed through transfer of a pair of electrons from reduced FAD (FADH2) to molecular oxygen or cytochrome c. The colour of FAD in stages 4 and 5 correspond to the colour of Erv1C30S and Erv1C130S respectively (the present study and [28]).