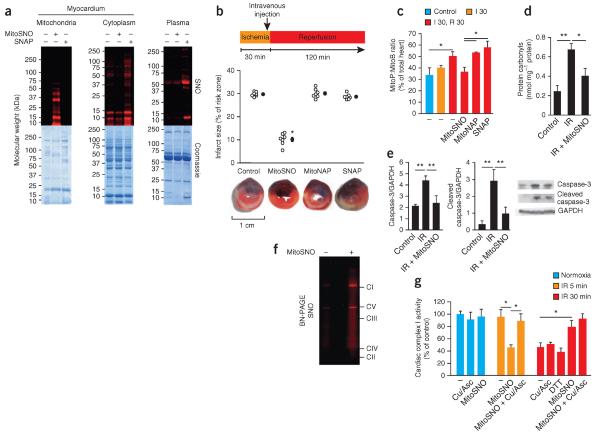
Figure 1.
S-nitrosation of mitochondrial proteins is required for S-nitrosation–mediated protection from cardiac ischemia-reperfusion injury. (a) Protein S-nitrosation in mitochondrial and cytosolic fractions of the myocardium, as well as in blood plasma, of mice after tail-vein injection of MitoSNO or SNAP just before reperfusion. Top, S-nitrosated proteins, as detected by selective reduction of protein S-nitrosothiols in the presence of a red fluorescent maleimide. Bottom, protein loading of the scanned gels (top) assessed by Coomassie staining. SNO, S-nitrosation. (b) Top, quantification of myocardial infarct size after tail-vein injection of MitoSNO, SNAP or MitoNAP. Each open circle represents data from a single mouse, and filled circles represent the mean values of all mice for a particular condition. Bottom, representative images of cross-sections from mouse hearts treated as indicated above. Infarcted tissue is white, the rest of the area at risk is red, and nonrisk tissue is dark blue. n = 7 mice per group. (c) Mitochondrial hydrogen peroxide measured in vivo by the selective oxidation of the mitochondria-targeted mass spectrometric probe MitoB to its product MitoP during myocardial ischemia-reperfusion. MitoSNO, SNAP or MitoNAP was injected at reperfusion, and control hearts were collected from mice without intervention. n = 3–6 mice per group. Control, 60 min of normoxic perfusion; I 30, 30-min ischemia; R 30, 30-min reperfusion. (d,e) Assessment of protein oxidative damage (as assessed by protein carbonyl formation, d) and apoptosis (as assessed by caspase-3 and cleaved caspase-3 amounts, e) after myocardial ischemia-reperfusion (IR) with or without mitochondrial S-nitrosation by MitoSNO. The representative western blots in e show the amounts of caspase-3 and cleaved caspase-3, along with the amounts of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control. n = 3 mice per group. (f) BN-PAGE analysis of heart mitochondria identifying S-nitrosated respiratory complexes in mice injected with MitoSNO during ischemia-reperfusion injury followed by S-nitrosothiol labeling. CI–CV indicate the locations of oxidative phosphorylation complexes I–V. (g) Complex I activity in vivo at baseline and after ischemia and reperfusion for 5 min (IR 5 min) or 30 min (IR 30 min) with or without MitoSNO injection. Following mitochondrial isolation from hearts, the S-nitrosothiol-selective reductant Cu/Asc, or the general thiol reductant DTT, were added where indicated in vitro to test for the reversibility of complex I inhibition. Complex I activity was normalized to citrate synthase activity. Cu/Asc, copper and ascorbate; DTT, dithiothreitol. n = 3 mice per group. *P < 0.05, **P < 0.01 determined by one-way analysis of variance (ANOVA). Data (c–e,g) are shown as the mean ± s.e.m. of at least three replicates.