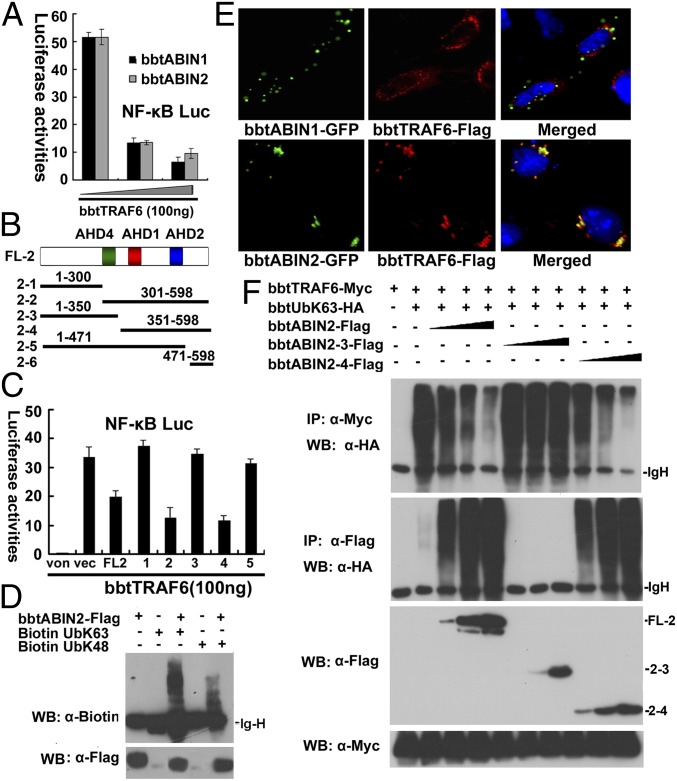
Fig. 3.
K63-linked ubiquitinated bbtTRAF6 is the target of bbtABIN2. (A) Luciferase reporter assays showed that both bbtABIN1 and bbtABIN2 can attenuate the NF-κB activation mediated by bbtTRAF6 in a dose-dependent manner in HEK 293T cells. Reporter experiments were conducted in triplicate; vertical bars indicate mean ± SD. Data are representative of three independent experiments. (B) Vectors of bbtABIN2 used in this study. (C) Luciferase reporter assays showed that truncated mutants containing the AHD2 region of bbtABIN2 can inhibit the bbtTRAF6-mediated activation of NF-κB. FL2 indicates the WT bbtABIN2; 1–6 indicate bbtABIN2-1 to bbtABIN2-6. von indicates the blank control; vec indicates the pcDNA3.1 vector. (D) In vitro binding assays showed that bbtABIN2 can bind both K63-linked and K48-linked ubiquitin chains. WB, Western blot. (E) Confocal analysis showed that bbtABIN2, but not bbtABIN1, can colocalize with bbtTRAF6. Original magnification 400×. (F) Both WT bbtABIN2 and the truncated mutant bbtABIN2-4 can bind to K63-linked polyubiquitin chains by competing with bbtTRAF6, whereas bbtABIN2-3, which lacks the UBAN domain, cannot. The abbreviation bbtUb indicates the WT amphioxus ubiquitin, whereas bbtUbK63 indicates the amphioxus ubiquitin mutant with substitution of arginine for all lysine residues except the lysine at position 63. All of the co-IP (IP) assays were done at least twice.